Comparison of Compressive Stress-Relaxation Behavior in Osteoarthritic (ICRS Graded) Human Articular Cartilage
Abstract
:1. Introduction
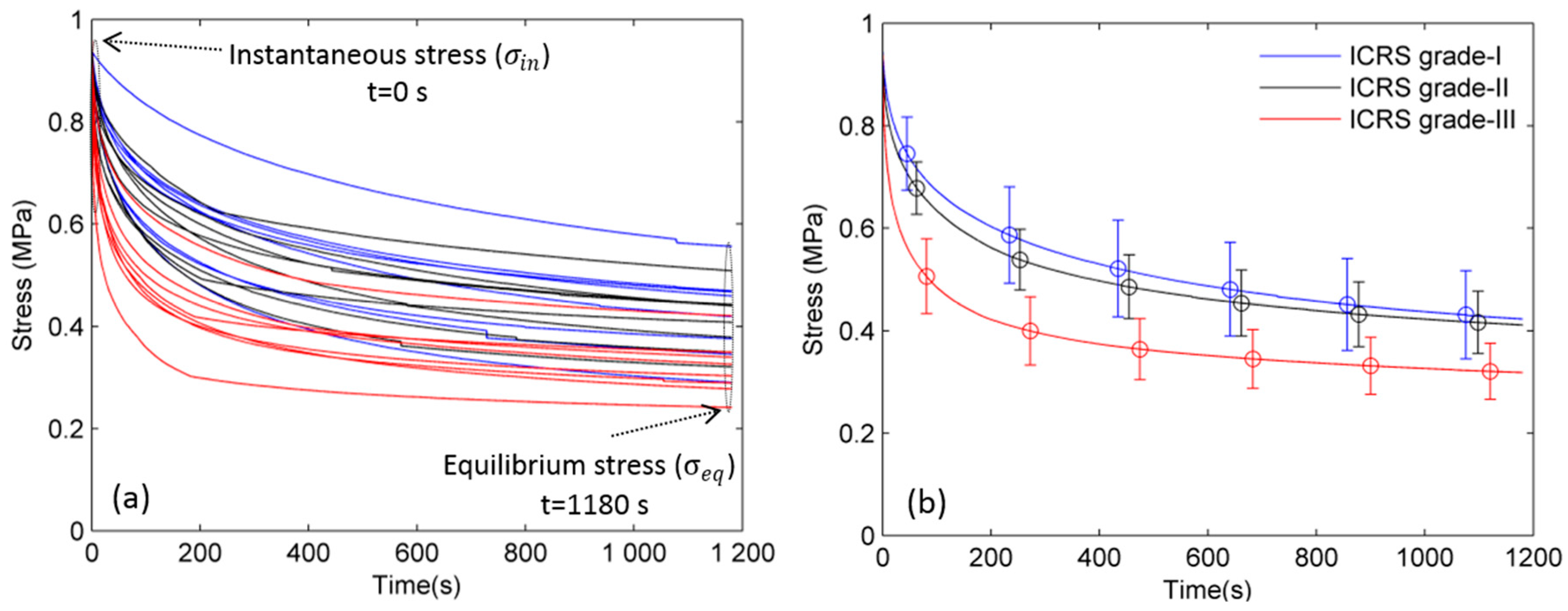
2. Results
2.1. Young’s Modulus
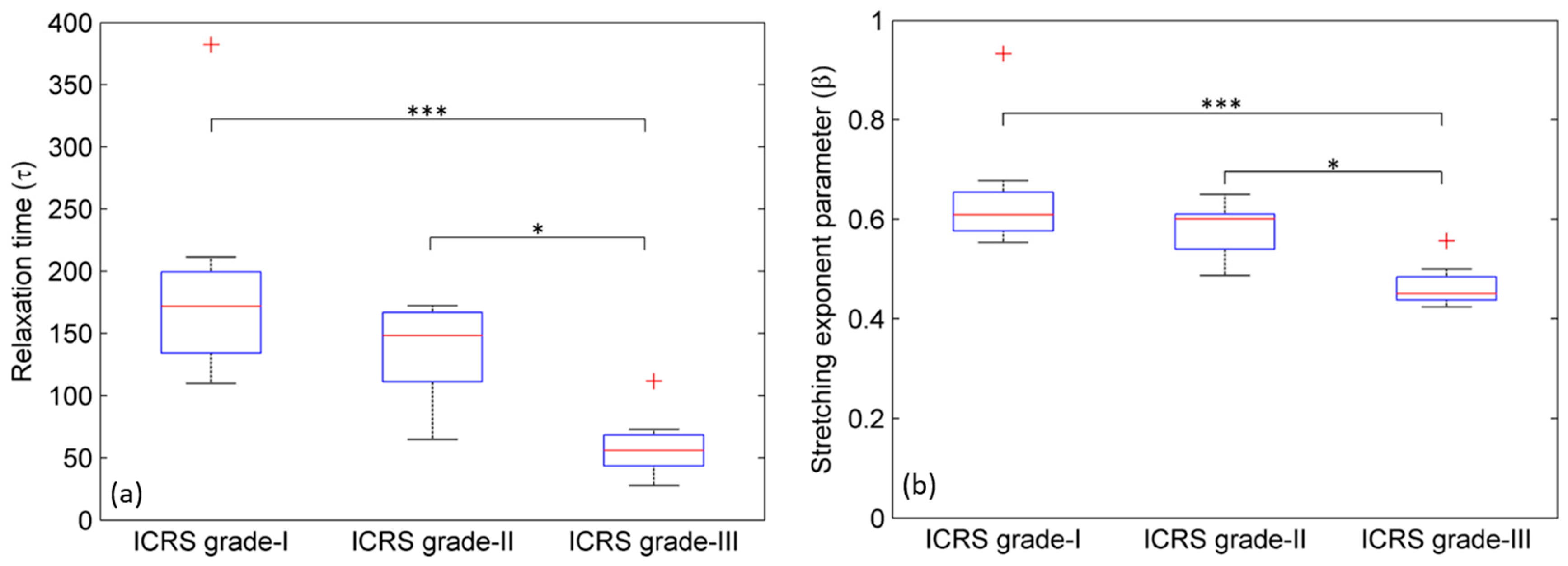
2.2. Stress Relaxation Time (τ)
2.3. Stretching Exponent Parameter (β)
3. Discussion
3.1. Young’s Modulus
3.2. Stress Relaxation Time (τ)
3.3. Stretching Exponent Parameter (β)
4. Materials and Methods
4.1. Sample Preparation
4.2. Data Acquisition and Analysis
4.3. Statistical Analyses
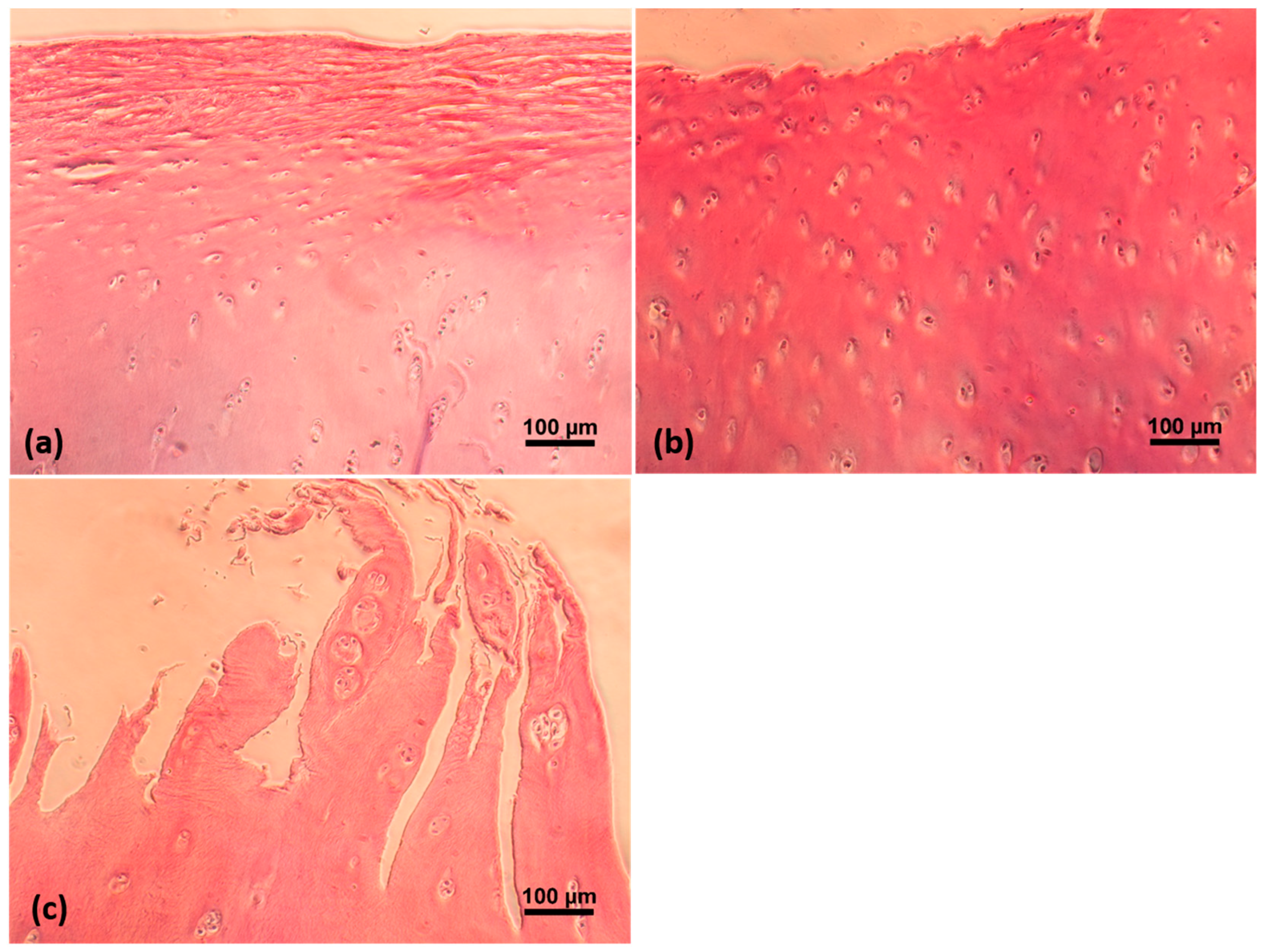
4.4. Histological Assessment
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Laasanen, M.S.; Toyras, J.; Korhonen, R.K.; Rieppo, J.; Saarakkala, S.; Nieminen, M.T.; Hirvonen, J.; Jurvelin, J.S. Biomechanical properties of knee articular cartilage. Biorheology 2003, 40, 133–140. [Google Scholar] [PubMed]
- Hamerman, D. Clinical implications of osteoarthritis and ageing. Ann. Rheum. Dis. 1995, 54, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Schofield, D.; Callander, E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014, 10, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Grønhaug, K.M.; Davies, C.L.; Drogset, J.O.; Lilledahl, M.B. Nonlinear optical microscopy of early stage (ICRS Grade-I) osteoarthritic human cartilage. Biomed. Opt. Express 2015, 6, 1895–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Grønhaug, K.M.; Afseth, N.K.; Isaksen, V.; Davies, C.L.; Drogset, J.O.; Lilledahl, M.B. Optical investigation of osteoarthritic human cartilage (ICRS Grade) by confocal Raman spectroscopy: A pilot study. Anal. Bioanal. Chem. 2015, 407, 8067–8077. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Grønhaug, K.M.; Romijn, E.I.; Drogset, J.O.; Lilledahl, M.B. Analysis of Human Knee Osteoarthritic Cartilage Using Polarization Sensitive Second Harmonic Generation Microscopy. Proc. SPIE 2014, 9129. [Google Scholar] [CrossRef]
- Kleemann, R.U.; Krocker, D.; Cedraro, A.; Tuischer, J.; Duda, G.N. Altered cartilage mechanics and histology in knee osteoarthritis: Relation to clinical assessment (ICRS Grade). Osteoarthr. Cartil. 2005, 13, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Temple-Wong, M.M.; Bae, W.C.; Chen, M.Q.; Bugbee, W.D.; Amiel, D.; Coutts, R.D.; Lotz, M.; Sah, R.L. Biomechanical, structural, and biochemical indices of degenerative and osteoarthritic deterioration of adult human articular cartilage of the femoral condyle. Osteoarthr. Cartil. 2009, 17, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Bradica, G.; Tria, A. Relationship among biomechanical, biochemical, and cellular changes associated with osteoarthritis. Crit. Rev. Biomed. Eng. 2001, 29, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Juras, V.; Bittsansky, M.; Majdisova, Z.; Szomolanyi, P.; Sulzbacher, I.; Gabler, S.; Stampfl, J.; Schuller, G.; Trattnig, S. In vitro determination of biomechanical properties of human articular cartilage in osteoarthritis using multi-parametric MRI. J. Magn. Reson. 2009, 197, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.; Villars, M.; Thibaud, S. Viscoelastic modeling and quantitative experimental characterization of normal and osteoarthritic human articular cartilage using indentation. J. Mech. Behav. Biomed. Mater. 2013, 24, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.; van Donkelaar, C.C.; van Rietbergen, R.; Huiskes, R. The role of computational models in the search for the mechanical behavior and damage mechanisms of articular cartilage. Med. Eng. Phys. 2005, 27, 810–826. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.P.; Houle, M.A.; Chen, M.; Price, A.J.; Légaré, F.; Gill, H.S. Damage initiation and progression in the cartilage surface probed by nonlinear optical microscopy. J. Mech. Behav. Biomed. Mater. 2012, 5, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Youn, I.; Guilak, F.; Setton, L.A. Compressive properties of mouse articular cartilage determined in a novel micro-indentation test method and biphasic finite element model. J. Biomech. Eng. 2006, 128, 766–771. [Google Scholar] [PubMed]
- Toyras, J.; Lyyra-Laitinen, T.; Niinimaki, M.; Lindgren, R.; Nieminen, M.T.; Kiviranta, I.; Jurvelin, J.S. Estimation of the Young’s modulus of articular cartilage using an arthroscopic indentation instrument and ultrasonic measurement of tissue thickness. J. Biomech. 2001, 34, 251–256. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Rosenwasser, M.P.; Buckwalter, J.A.; Malinin, T.I.; Mow, V.C. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J. Orthop. Res. 1991, 9, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Hayes, W.C.; Mockros, L.F. Viscoelastic properties of human articular cartilage. J. Appl. Physiol. 1971, 31, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Mats Brittberg, C.; Aglietti, P.; Gambardella, R.; Hungary, L.H.; Hauselmann, H.J.; Jakon, R.P.; Levine, D.; Lohmander, S.; Mandelbaum, B.R.; Peterson, L.; et al. ICRS Cartilage Injury Evaluation Package. In Proceedings of the 3rd ICRS Meeting, Göteborg, Sweden, 28 April 2000; Available online: https://cartilage.org/content/uploads/2014/10/ICRS_evaluation.pdf (accessed on 29 January 2018).
- June, R.K.; Fyhrie, D.P. Enzymatic digestion of articular cartilage results in viscoelasticity changes that are consistent with polymer dynamics mechanisms. Biomed. Eng. Online 2009, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- June, R.K.; Fyhrie, D.P. A comparison of cartilage stress-relaxation models in unconfined compression: QLV and stretched exponential in combination with fluid flow. Comput. Methods Biomech. Biomed. Eng. 2013, 16, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Siegel, J.; Webb, S.E.; Leveque-Fort, S.; Cole, M.J.; Jones, R.; Dowling, K.; Lever, M.J.; French, P.M. Application of the stretched exponential function to fluorescence lifetime imaging. Biophys. J. 2001, 81, 1265–1274. [Google Scholar] [CrossRef]
- Szymanski, C. Investigation of Photophysical and Photochemical Processes in Conjugated Polymer Nanoparticles by Single particle and Ensemble Spectroscopy. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2009. [Google Scholar]
- Kempson, G.E.; Muir, H.; Swanson, S.A.; Freeman, M.A. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochim. Biophys. Acta 1970, 215, 70–77. [Google Scholar] [CrossRef]
- Akizuki, S.A.; Mow, V.C.; Lai, W.M.; Pita, J.; Howell, D.S. Topographical variations of the biphasic indentation properties of human tibial plateau cartilage. Trans. Orthop. Res. Soc. 1986, 11, 10–19. [Google Scholar]
- Mansour, J.M. Biomechanics of cartilage. In Kinesiology: The Mechanics and Pathomechanics of Human Movement, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 69–83. [Google Scholar]
- Brown, C.P.; Crawford, R.W.; Oloyede, A. An alternative mechanical parameter for assessing the viability of articular cartilage. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2009, 223, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kos, P.; Varga, F.; Handl, M.; Kautzner, J.; Chudáček, V.; Držík, M.; Povýšil, C.; Trč, T.; Amler, E.; Hanus, M. Correlation of dynamic impact testing, histopathology and visual macroscopic assessment in human osteoarthritic cartilage. Int. Orthop. SICOT 2011, 35, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, C.A.; Muir, H. Biochemical changes in the cartilage of the knee in experimental and natural osteoarthritis in the dog. J. Bone Jt. Surg. 1976, 58, 94–101. [Google Scholar]
- Lane, J.M.; Chisena, E.; Black, J. Experimental knee instability: Early mechanical property changes in articular cartilage in a rabbit model. Clin. Orthop. Relat. Res. 1979, 140, 262–265. [Google Scholar] [CrossRef]
- Doi, M.; Edwards, S.F. The Theory of Polymer Dynamics; Clarendon, Oxford University Press: New York, NY, USA, 1986. [Google Scholar]
- Van Wart, H.E.; Steinbrink, D.R. Complementary substrate specificities of class I and class II collagenases from Clostridium histolyticum. Biochemistry 1985, 24, 6520–6526. [Google Scholar] [CrossRef] [PubMed]
- Ohya, T.; Kaneko, Y. Novel hyaluronidase from streptomyces. Biochim. Biophys. Acta 1970, 198, 607–609. [Google Scholar] [CrossRef]
- Fyhrie, D.P.; Barone, J.R. Polymer dynamics as a mechanistic model for the flow-independent viscoelasticity of cartilage. J. Biomech. Eng. 2003, 125, 578–584. [Google Scholar] [PubMed]
- Ruberti, J.W.; Sokoloff, J.B. Theory of the short time mechanical relaxation in articular cartilage. J. Biomech. Eng. 2011, 133, 104504. [Google Scholar] [PubMed]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [PubMed]


| Sample | Stress-Relaxation Time Constant (τ) | Instantaneous Young’s Modulus i.e., Yin (at t = 0 s) | Equilibrium Young’s Modulus i.e., Yeq (at t = 1180 s) | Ratio (Yin/Yeq) | Stretching Exponent (β) | Histological Grade (OARSI) |
|---|---|---|---|---|---|---|
| ICRS grade-I | ||||||
| #Section-1 | 211.2 | 6.816 | 6.816 | 2.236544 | 0.6776 | 1 |
| #Section-4 | 136 | 3.426691 | 3.426691 | 3.228219 | 0.6013 | 2 |
| #Section-9 | 382.4 | 4.559351 | 4.559351 | 1.684617 | 0.9331 | 1 |
| #Section-11 | 157.6 | 3.7488 | 3.7488 | 2.005482 | 0.5947 | 2 |
| #Section-13 | 187.6 | 5.797113 | 5.797113 | 1.996035 | 0.631 | 1 |
| #Section-16 | 185.9 | 3.360478 | 3.360478 | 2.039817 | 0.6165 | 2 |
| #Section-19 | 110 | 4.172465 | 4.172465 | 2.493802 | 0.5537 | 1 |
| #Section-21 | 132.5 | 3.910431 | 3.910431 | 2.713876 | 0.5578 | 1 |
| ICRS grade-II | ||||||
| #Section-3 | 116.4 | 3.997535 | 3.997535 | 2.920333 | 0.6004 | 2 |
| #Section-5 | 172.3 | 5.50605 | 5.50605 | 2.475084 | 0.6501 | 2 |
| #Section-7 | 151.3 | 4.317236 | 4.317236 | 2.681396 | 0.6163 | 1 |
| #Section-12 | 64.82 | 4.065479 | 4.065479 | 2.297605 | 0.4867 | 2 |
| #Section-14 | 172 | 3.928383 | 3.928383 | 2.129691 | 0.5743 | 2 |
| #Section-17 | 106.1 | 3.736842 | 3.736842 | 2.126649 | 0.5062 | 2 |
| #Section-20 | 145.3 | 3.421194 | 3.421194 | 2.116525 | 0.6046 | 2 |
| #Section-24 | 161.3 | 3.685106 | 3.685106 | 1.842395 | 0.6004 | 1 |
| ICRS grade-III | ||||||
| #Section-2 | 64.28 | 1.497124 | 0.443423 | 3.376287 | 0.4393 | 3 |
| #Section-6 | 43.52 | 2.203448 | 0.712712 | 3.091636 | 0.4363 | 3 |
| #Section-8 | 55.04 | 4.301901 | 1.327796 | 3.239881 | 0.4687 | 4 |
| #Section-10 | 56.87 | 1.687635 | 0.586799 | 2.876001 | 0.4422 | 3 |
| #Section-15 | 43.9 | 2.677714 | 0.997392 | 2.684714 | 0.4584 | 3 |
| #Section-18 | 111.6 | 2.870444 | 1.288061 | 2.228499 | 0.5566 | 3 |
| #Section-22 | 72.66 | 3.397942 | 1.231759 | 2.758608 | 0.4996 | 4 |
| #Section-23 | 27.74 | 1.927954 | 0.496422 | 3.883696 | 0.4236 | 3 |
| Cartilage Section (Total n = 24) | Assignment of ICRS Grdae | ||
|---|---|---|---|
| Grade-I (n = 8) | Grade-II (n = 8) | Grade-III (n = 8) | |
| Patient 1 | Section-1 (L) | x | x |
| Patient 2 | x | x | Section-2 (M) |
| Patient 3 | x | Section-3 (L) | x |
| Patient 4 | Section-4 (M) | Section-5 (M) | Section-6 (L) |
| Patient 5 | x | Section-7 (M) | Section-8 (L) |
| Patient 6 | Section-9 (L) | x | Section-10 (M) |
| Patient 7 | Section-11 (L) | Section-12 (M) | x |
| Patient 8 | Section-13 (L) | Section-14 (L) | Section-15 (M) |
| Patient 9 | Section-16 (M) | x | x |
| Patient 10 | x | Section-17 (M) | Section-18 (M) |
| Patient 11 | Section-19 (L) | Section-20 (M) | x |
| Patient 12 | Section-21 (L) | x | Section-22 (M) |
| Patient 13 | x | x | Section-23 (M) |
| Patient 14 | x | Section-24 (L) | x |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Pierce, D.M.; Isaksen, V.; Davies, C.D.L.; Drogset, J.O.; Lilledahl, M.B. Comparison of Compressive Stress-Relaxation Behavior in Osteoarthritic (ICRS Graded) Human Articular Cartilage. Int. J. Mol. Sci. 2018, 19, 413. https://doi.org/10.3390/ijms19020413
Kumar R, Pierce DM, Isaksen V, Davies CDL, Drogset JO, Lilledahl MB. Comparison of Compressive Stress-Relaxation Behavior in Osteoarthritic (ICRS Graded) Human Articular Cartilage. International Journal of Molecular Sciences. 2018; 19(2):413. https://doi.org/10.3390/ijms19020413
Chicago/Turabian StyleKumar, Rajesh, David M. Pierce, Vidar Isaksen, Catharina De Lange Davies, Jon O. Drogset, and Magnus B. Lilledahl. 2018. "Comparison of Compressive Stress-Relaxation Behavior in Osteoarthritic (ICRS Graded) Human Articular Cartilage" International Journal of Molecular Sciences 19, no. 2: 413. https://doi.org/10.3390/ijms19020413





