Changes in the Distribution of Cocaine- and Amphetamine-Regulated Transcript-Containing Neural Structures in the Human Colon Affected by the Neoplastic Process
Abstract
:1. Introduction
2. Results
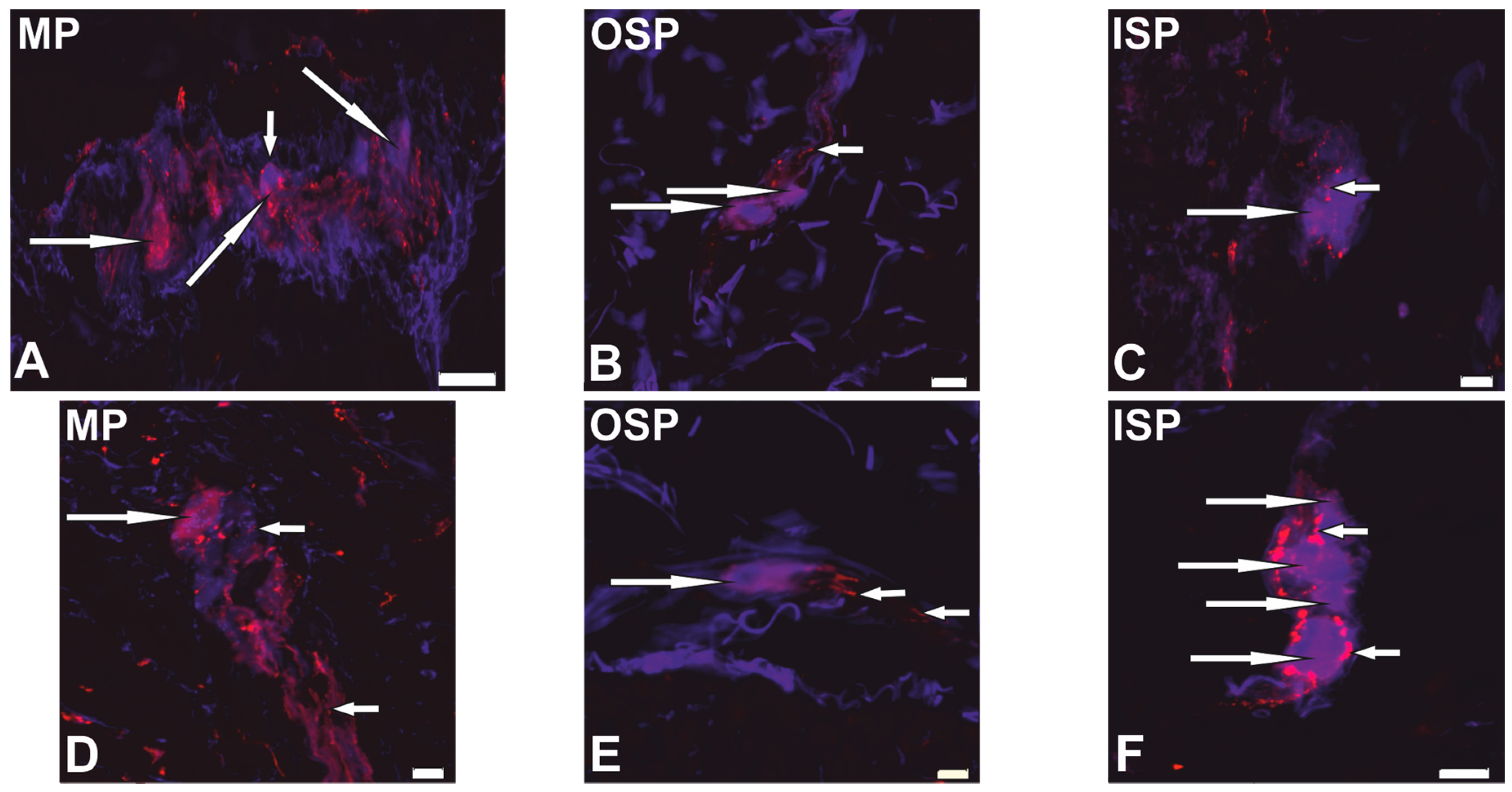
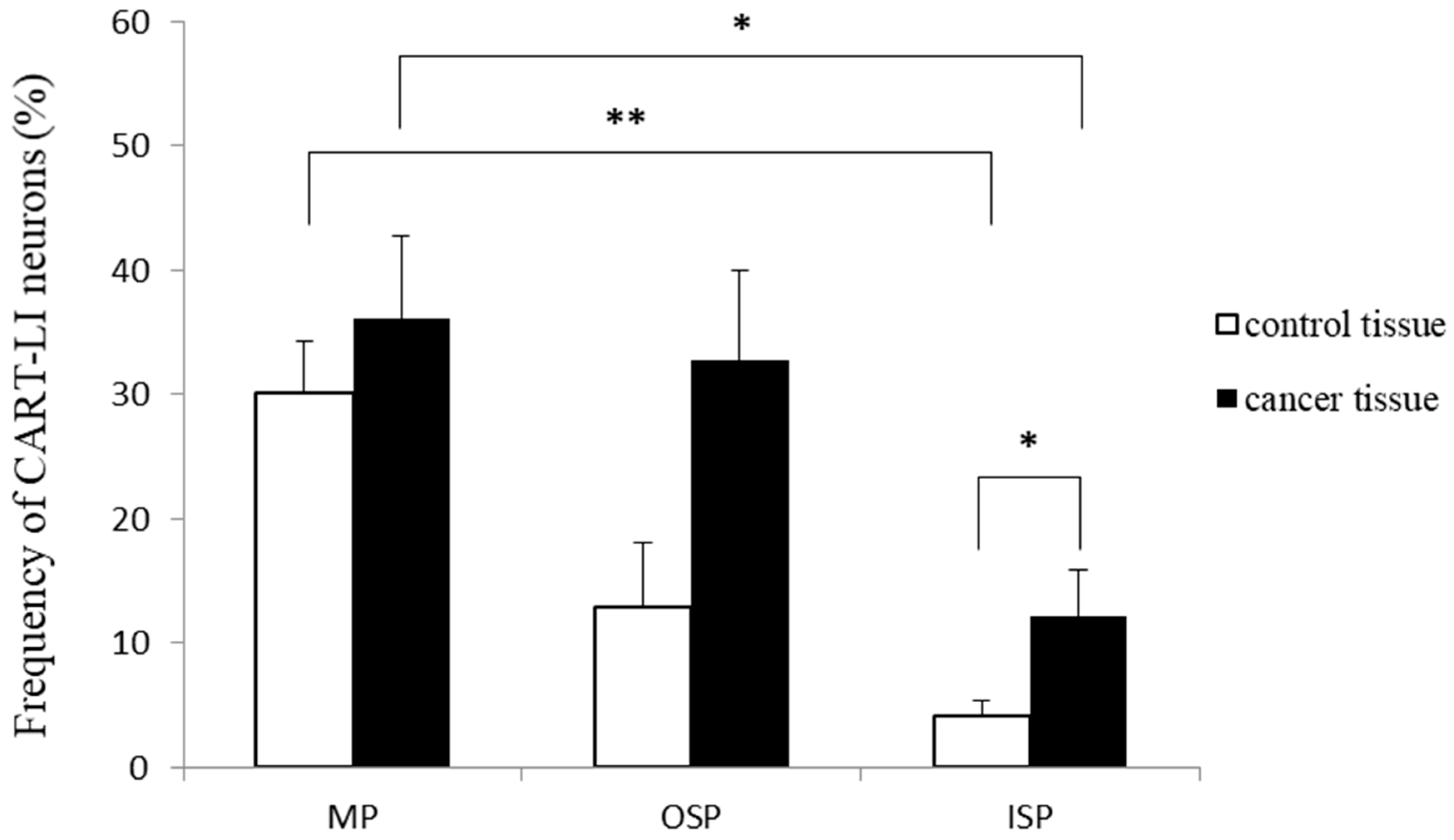
2.1. Neurochemical Phenotype of Neurons from Studied Myenteric and Submucous Plexuses
2.2. Distribution Patterns of Nerve Fibers Containing CART in Muscle Layers of the Colonic Wall
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment
4.2. Tissue Preparation
4.3. Double-Labelling Immunofluorescence
4.4. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Browning, K.N.; Travagli, R.A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol. 2014, 4, 1339–1368. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef]
- Kyösola, K.; Rechardt, L.; Veijola, L.; Waris, T.; Penttilä, O. Innervation of the human gastric wall. J. Anat. 1980, 131, 453–470. [Google Scholar] [PubMed]
- Timmermans, J.P.; Adriaensen, D.; Cornelissen, W.; Scheuermann, D.W. Structural organization and neuropeptide distribution in the mammalian enteric nervous system, with special attention to those components involved in mucosal reflexes. Comp. Biochem. Physiol. 1997, 118, 331–340. [Google Scholar] [CrossRef]
- Wedel, T.; Roblick, U.; Gleiss, J.; Schiedeck, T.; Bruch, H.P.; Kühnel, W.; Krammer, H.J. Organization of the enteric nervous system in the human colon demonstrated by wholemount immunohistochemistry with special reference to the submucous plexus. Ann. Anat. 1999, 181, 327–337. [Google Scholar] [CrossRef]
- Crowe, R.; Kamm, M.A.; Burnstock, G.; Lennard-Jones, J.E. Peptide-containing neurons in different regions of the submucous plexus of human sigmoid colon. Gastroenterology 1992, 102, 461–467. [Google Scholar] [CrossRef]
- Furness, J.B. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 2000, 81, 87–96. [Google Scholar] [CrossRef]
- Hansen, M.B. The enteric nervous system I: Organisation and classification. Pharmacol. Toxicol. 2003, 92, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B. The enteric nervous system II: Gastrointestinal functions. Pharmacol. Toxicol. 2003, 92, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Spiess, J.; Villarreal, J.; Vale, W. Isolation and sequence analysis of a somatostatin-like polypeptide from ovine hypothalamus. Biochemistry 1981, 20, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.; McKinzie, A.A.; Couceyro, P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J. Neurosci. 1995, 15, 2471–2481. [Google Scholar] [PubMed]
- Dun, N.J.; Dun, S.L.; Kwok, E.H.; Yang, J.; Chang, J. Cocaine- and amphetamine-regulated transcript-immunoreactivity in the rat sympatho-adrenal axis. Neurosci. Lett. 2000, 283, 97–100. [Google Scholar] [CrossRef]
- Couceyro, P.; Paquet, M.; Koylu, E.; Kuhar, M.J.; Smith, Y. Cocaine- and amphetamine-regulated transcript (CART) peptide immunoreactivity in myenteric plexus neurons of the rat ileum and co-localization with choline acetyltransferase. Synapse 1998, 30, 1–8. [Google Scholar] [CrossRef]
- Jensen, P.B.; Kristensen, P.; Clausen, J.T.; Judge, M.E.; Hastrup, S.; Thim, L.; Wulff, B.S.; Foged, C.; Jensen, J.; Holst, J.J.; et al. The hypothalamic satiety peptide CART is expressed in anorectic and non-anorectic pancreatic islets tumors an in the normal islets of Langerhans. FEBS Lett. 1999, 447, 139–143. [Google Scholar] [CrossRef]
- Ekblad, E.; Kuhar, M.; Wierup, N.; Sundler, F. Cocaine- and amphetamine-regulated transcript: Distribution and function in rat gastrointestinal tract. Neurogastroenterol. Motil. 2003, 15, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Wierup, N.; Gunnarsdóttir, A.; Ekblad, E.; Sundler, F. Characterisation of CART-containing neurons and cells in the porcine pancreas, gastro-intestinal tract, adrenal and thyroid glands. BMC Neurosci. 2007, 8, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ekblad, E. CART in the enteric nervous system. Peptides 2006, 27, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Han, L.; Xu, Y. Roles of cocaine- and amphetamine-regulated transcript in the central nervous system. Clin. Exp. Pharmacol. Physiol. 2012, 39, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Bulc, M.; Gonkowski, S.; Landowski, P.; Kamińska, B.; Całka, J. Immunohistochemical distribution of cocaine and amphetamine regulatory peptide-lika immunoreactive (CART-LI) nerve fibers in the circular muscle layer and their relationship to other peptides in the human caecum. Acta Histochem. 2014, 116, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Bulc, M.; Gonkowski, S.; Landowski, P.; Kamińska, B.; Całka, J. Immunohistochemical evidence of the co-localisation of cocaine and amphetamine regulatory peptide with neuronal isoform of nitric oxide synthase, vasoactive intestinal peptide and galanin within the circular muscle layer of the human caecum. Folia Morphol. 2015, 74, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Kamińska, B.; Landowski, P.; Całka, J. Immunohistochemical distribution of cocaine- and amphetamine-regulated transcript peptide-like immunoreactive (CART-LI) nerve fibers and various degree of co-localization with other neuronal factors in the circular muscle layer of human descending colon. Histol. Histopathol. 2013, 28, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Lomax, A.E.; Fernandez, E.; Sharkey, K.A. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol. Motil. 2005, 17, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Moynes, D.M.; Graydon, H.L.; Beyak, M.J.; Lomax, A.E. Effects of inflammation on the innervation of the colon. Toxicol. Pathol. 2014, 42, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, J. Morphological changes in the enteric nervous system caused by carcinoma of the human large intestine. Folia Histochem. Cytobiol. 2010, 48, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, J.; Łakomy, I.M. Changes in vasoactive intestinal peptide, pituitary adenylate cyclase-activating polypeptide and neuropeptide Y-ergic structures of the enteric nervous system in the carcinoma of the human large intestine. Folia Histochem. Cytobiol. 2010, 48, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, J.; Pidsudko, Z. Characteristic of galaninergic components of the enteric nervous system in the cancer invasion of human large intestine. Ann. Anat. 2012, 194, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, J.; Kaleczyc, J. Somatostatin, substance P and calcitonin gene-related peptide-positive intramural nerve structures of the human large intestine affected by carcinoma. Folia Histochem. Cytobiol. 2010, 48, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, P.; Godlewski, J.; Kieżun, J.; Kraziński, B.E.; Kmieć, Z. Colorectal cancer patients exhibit increased levels of galanin in serum and colon tissues. Oncol. Lett. 2016, 12, 3323–3329. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Kamińska, B.; Burliński, P.; Kroll, A.; Całka, J. The influence of drug-resistant ulcerative colitis on the number of cocaine- and amphetamine-regulated transcript peptide-like immunoreactive (CART-LI) mucosal nerve fibers of the descending colon in children. Przegląd Gastroenterologiczny 2009, 4, 147–151. [Google Scholar]
- Landerholm, K.; Falkmer, S.E.; Jarhult, J.; Sundler, F.; Wierup, N. Cocaine- and amphetamine-regulated transcript in neuroendocrine tumors. Neuroendocrinology 2011, 94, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Landerholm, K.; Shcherbina, L.; Falkmer, S.E.; Jarhult, J.; Wierup, N. Expression of cocaine- and amphetamine-regulated transcript is associated with worse survival in small bowel carcinoid tumors. Clin. Cancer Res. 2012, 18, 3668–3676. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Gonkowski, S. Cocaine- and amphetamine-regulated transcript (CART) peptide in mammals gastrointestinal system. Ann. Anim. Sci. 2017, 17, 3–21. [Google Scholar] [CrossRef]
- Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics 2011, 11, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Wojtkiewicz, J.; Gonkowski, S.; Bladowski, M.; Majewski, M. Characterisation of cocaine- and amphetamine-regulated transcript-like immunoreactive (CART-LI) enteric neurons in the porcine small intestine. Acta Vet. Hung. 2012, 60, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Burliński, P.; Skobowiat, C.; Majewski, M.; Arciszewski, M.B.; Radziszewski, P.; Całka, J. Distribution of cocaine- and amphetamine-regulated transcript-like immunoreactive (CART-LI) nerve structures in the porcine large intestine. Acta Vet. Hung. 2009, 57, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Kasacka, I.; Piotrowska, Z.; Car, H.; Janiuk, I.; Lebkowski, W. Cocaine- and amphetamine-regulated transcript: Identification and distribution in human gastrointestinal tract. J. Biol. Regul. Homeost. Agents 2012, 26, 419–428. [Google Scholar] [PubMed]
- Margolis, K.G.; Gershon, M.D. Neuropeptides and inflammatory bowel disease. Curr. Opin. Gastroenterol. 2009, 25, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Saffrey, M.J. Cellular changes in the enteric nervous system during ageing. Dev. Biol. 2013, 382, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, B.; Srinivasan, S. Diabetes and the enteric nervous system. Neurogastroenterol. Motil. 2007, 19, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Gonkowski, S.; Zielonka, Ł.; Dąbrowski, M.; Całka, J. T2 toxin-induced changes in cocaine- and amphetamine-regulated transcript (CART)-like immunoreactivity in the enteric nervous system within selected fragments of the porcine digestive tract. Neurotox. Res. 2017, 31, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsdóttir, A.; Wierup, A.; Larsson, L.T.; Kuhar, M.J.; Ekblad, E. CART-peptide immunoreactivity in enteric nerves in patients with Hirschprung’s disease. Eur. J. Pediatr. Surg. 2007, 17, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Bech, P.; Winstanley, V.; Murphy, K.G.; Sam, A.H.; Meeran, K.; Ghatei, M.A.; Bloom, S.R. Elevated cocaine- and amphetamine-regulated transcript immunoreactivity in the circulation of patients with neuroendocrine malignancy. J. Clin. Endocrinol. Metab. 2008, 93, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Rogge, G.; Jones, D.; Hubert, G.W.; Lin, Y.; Kuhar, M.J. CART peptides: Regulators of body weight, reward and other functions. Nat. Rev. Neurosci. 2008, 9, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Tebbe, J.J.; Ortmann, E.; Schumacher, K.; Mönnikes, H.; Kobelt, P.; Arnold, R.; Schäfer, M.K. Cocaine- and amphetamine-regulated transcript stimulates colonic motility via central CRF receptor activation and peripheral cholinergic pathways in fed, conscious rats. Neurogastroenterol. Motil. 2004, 16, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Yamada, H.; Motomura, W.; Kohgo, Y. Cocaine- and amphetamine-regulated transcript (CART) acts in the central nervous system to inhibit gastric acid secretion via brain corticotrophin-releasing factor system. Endocrinology 2000, 141, 2854–2860. [Google Scholar] [CrossRef] [PubMed]
- Bharne, A.P.; Upadhya, M.A.; Shelkar, G.P.; Singru, P.S.; Subhedar, N.K.; Kokare, D.M. Neuroprotective effect of cocaine- and amphetamine-regulated transcript peptide in spinal cord injury in mice. Neuropharmacology 2013, 67, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Bulc, M.; Gonkowski, S.; Całka, J. Expression of cocaine- and amphetamine-regulated transcript (CART) in the porcine intramural neurons of stomach in the course of experimentally induced diabetes mellitus. J. Mol. Neurosci. 2015, 57, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Meshul, C.K.; Thuillier, P.; Goldberg, N.R.S.; Reddy, P.H. CART peptide is a potential endogenous antioxidant and preferentially localized in mitochondria. PLoS ONE. 2012, 7, e29343. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shen, H.; Liu, H.S.; Yu, S.J.; Reiner, D.J.; Harvey, B.K.; Hoffer, B.J.; Yang, Y.; Wang, Y. CART peptide induces neuroregeneration in stroke rats. J. Cereb. Blood Flow Metab. 2013, 33, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Risold, P.Y.; Bernard-Franchi, G.; Collard, C.; Jacquemard, C.; La Roche, A.; Griffond, B. Ontogenetic expression of CART-peptides in the central nervous system and the periphery: A possible neurotrophic role? Peptides 2006, 27, 1938–1941. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]

| “Kind” of Ganglionated Plexuses | Control Tissue from Operative Margin | Cancer Affected Area | p Value |
|---|---|---|---|
| MP | 98.7 ± 18.0 | 73.4 ± 15.3 | 0.56 |
| OSP | 12.0 ± 6.7 * | 12.5 ± 5.6 | 0.83 |
| ISP | 24.7 ± 6.9 ** | 28.4 ± 9.4 | 0.68 |
| Muscle Layer of Intestinal Wall | Control Tissue from Operative Margin | Cancer Affected Area |
|---|---|---|
| LM | 0.011 ± 0.002 | 0.014 ± 0.002 |
| CM | 0.031 ± 0.004 a | 0.036 ± 0.006 b |
| MM | 0.046 ± 0.014 a | 0.034 ± 0.008 |
| Antibody | Species | Catalog Number | Company | Dilution |
|---|---|---|---|---|
| Primary antibody | ||||
| CART (61–102) | rabbit | H-003-61 | Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA | 1:6000 |
| PGP 9.5 | mouse | 7863-2004 | Biogenesis, Kingstone, NH, USA | 1:2100 |
| Secondary antibody | ||||
| Polyclonal Goat Anti-Rabbit Immunoglobulins/Biotinylated | E0432 | Dako, Glostrup, DK | 1:1000 | |
| AMCA-conjugated donkey anti-mouse IgG | 715-156-151 | Jackson Immunoresearch, West Grove, PA, USA | 1:90 | |
| Cy™3-conjugated streptavidin | 016-160-084 | Jackson Immunoresearch, West Grove, PA, USA | 1:9000 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oponowicz, A.; Kozłowska, A.; Gonkowski, S.; Godlewski, J.; Majewski, M. Changes in the Distribution of Cocaine- and Amphetamine-Regulated Transcript-Containing Neural Structures in the Human Colon Affected by the Neoplastic Process. Int. J. Mol. Sci. 2018, 19, 414. https://doi.org/10.3390/ijms19020414
Oponowicz A, Kozłowska A, Gonkowski S, Godlewski J, Majewski M. Changes in the Distribution of Cocaine- and Amphetamine-Regulated Transcript-Containing Neural Structures in the Human Colon Affected by the Neoplastic Process. International Journal of Molecular Sciences. 2018; 19(2):414. https://doi.org/10.3390/ijms19020414
Chicago/Turabian StyleOponowicz, Agnieszka, Anna Kozłowska, Sławomir Gonkowski, Janusz Godlewski, and Mariusz Majewski. 2018. "Changes in the Distribution of Cocaine- and Amphetamine-Regulated Transcript-Containing Neural Structures in the Human Colon Affected by the Neoplastic Process" International Journal of Molecular Sciences 19, no. 2: 414. https://doi.org/10.3390/ijms19020414





