Loss of BID Delays FASL-Induced Cell Death of Mouse Neutrophils and Aggravates DSS-Induced Weight Loss
Abstract
:1. Introduction
2. Results
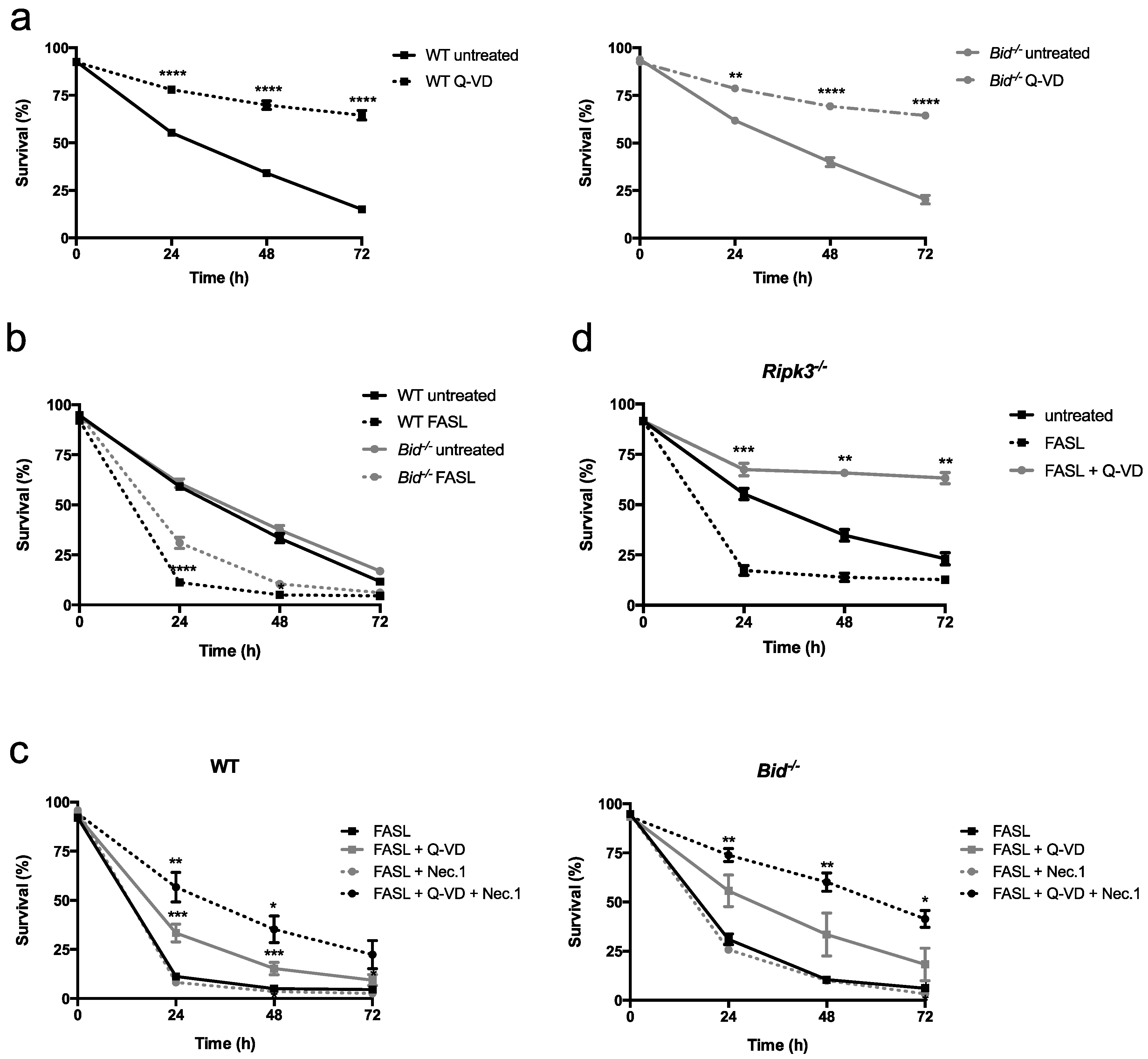
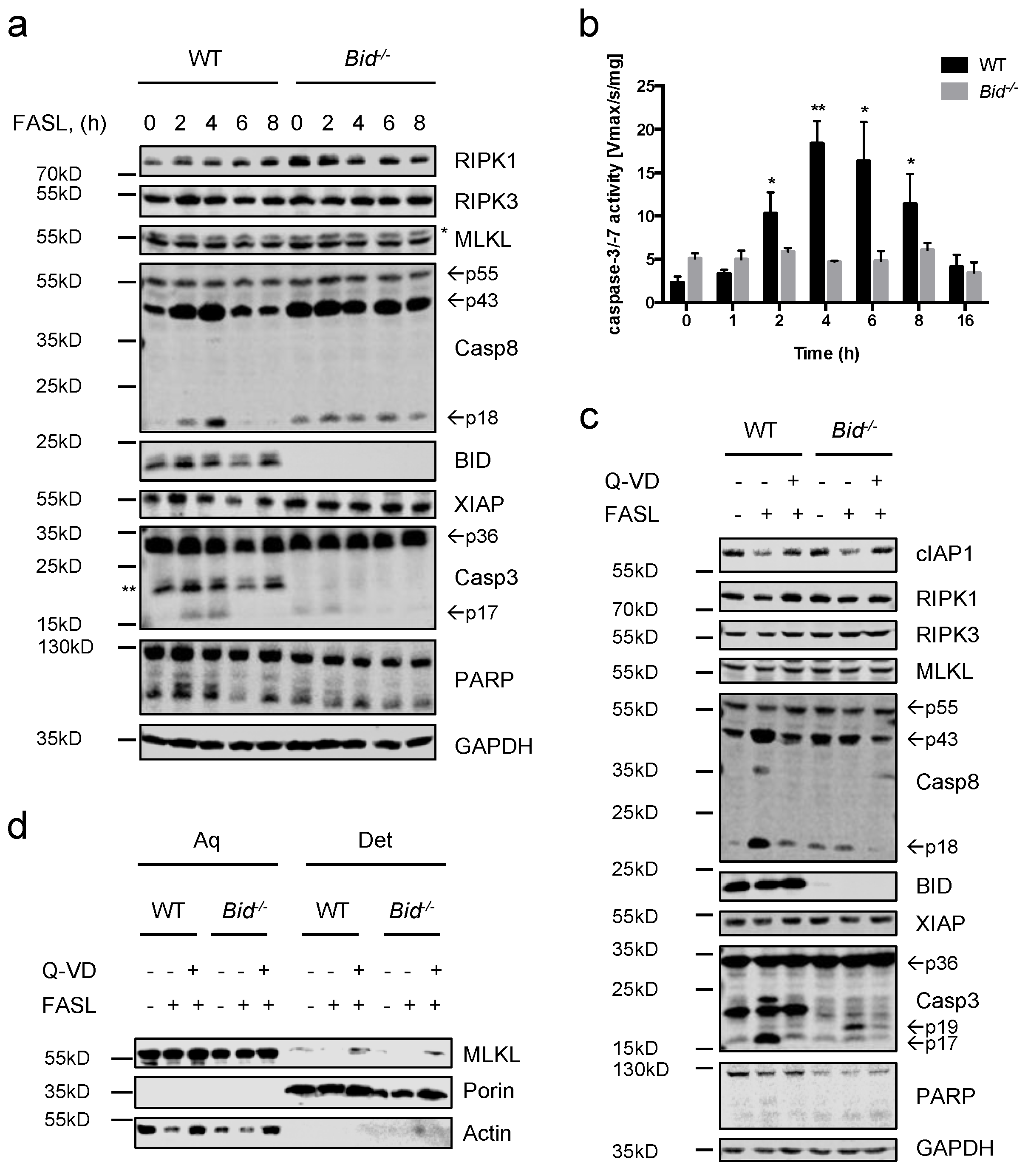
2.1. Fas Ligand (FASL) Induces Neutrophil Death, Which Is Delayed in Absence of BH3 Interacting Domain Death Agonist (BID)
2.2. Neutrophils Undergo Apoptosis in Response to FASL, Which Switches to Necroptosis Upon Inhibition of Caspases
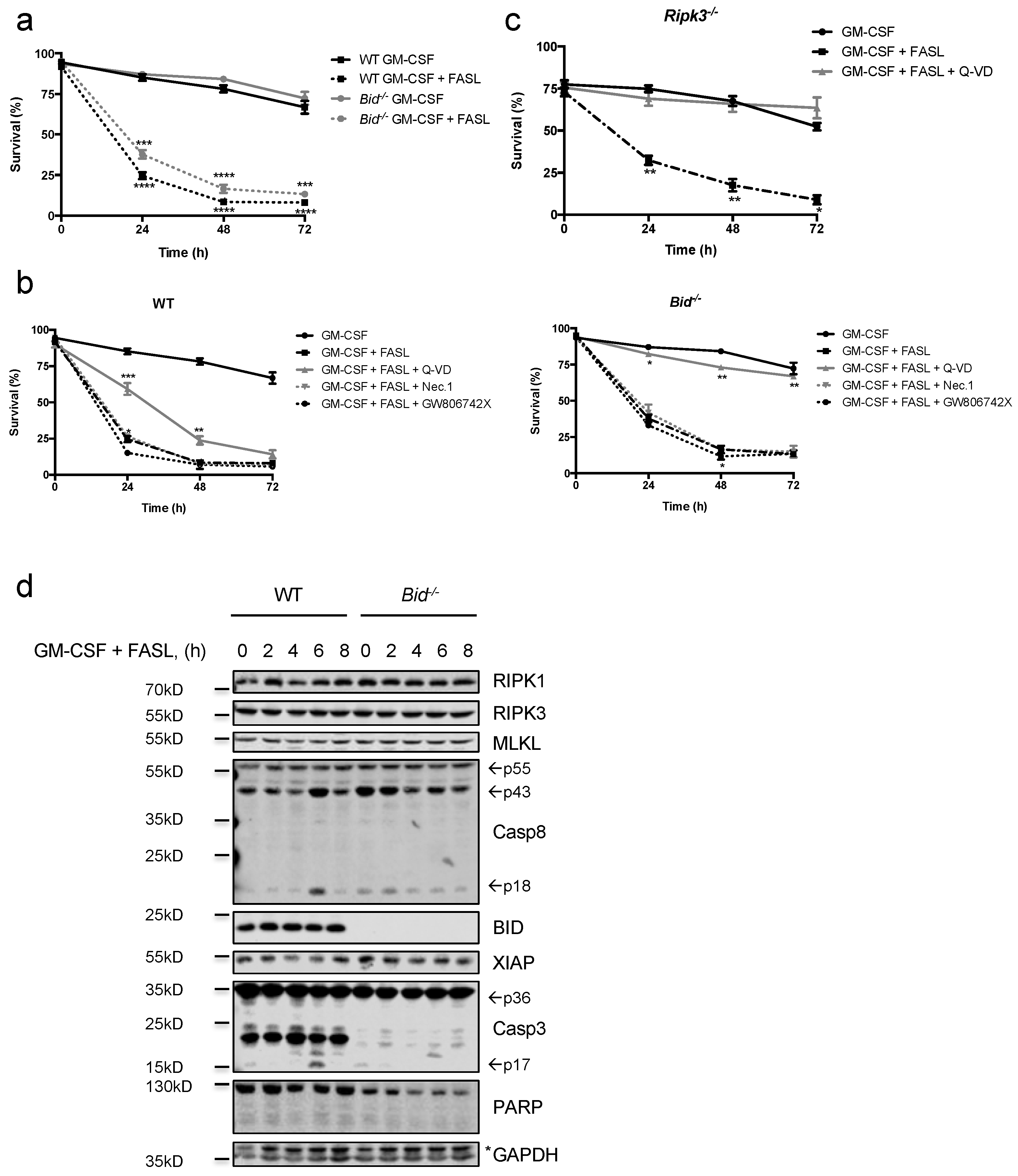
2.3. FASL Efficiently kills Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)- and LPS-Primed Neutrophils
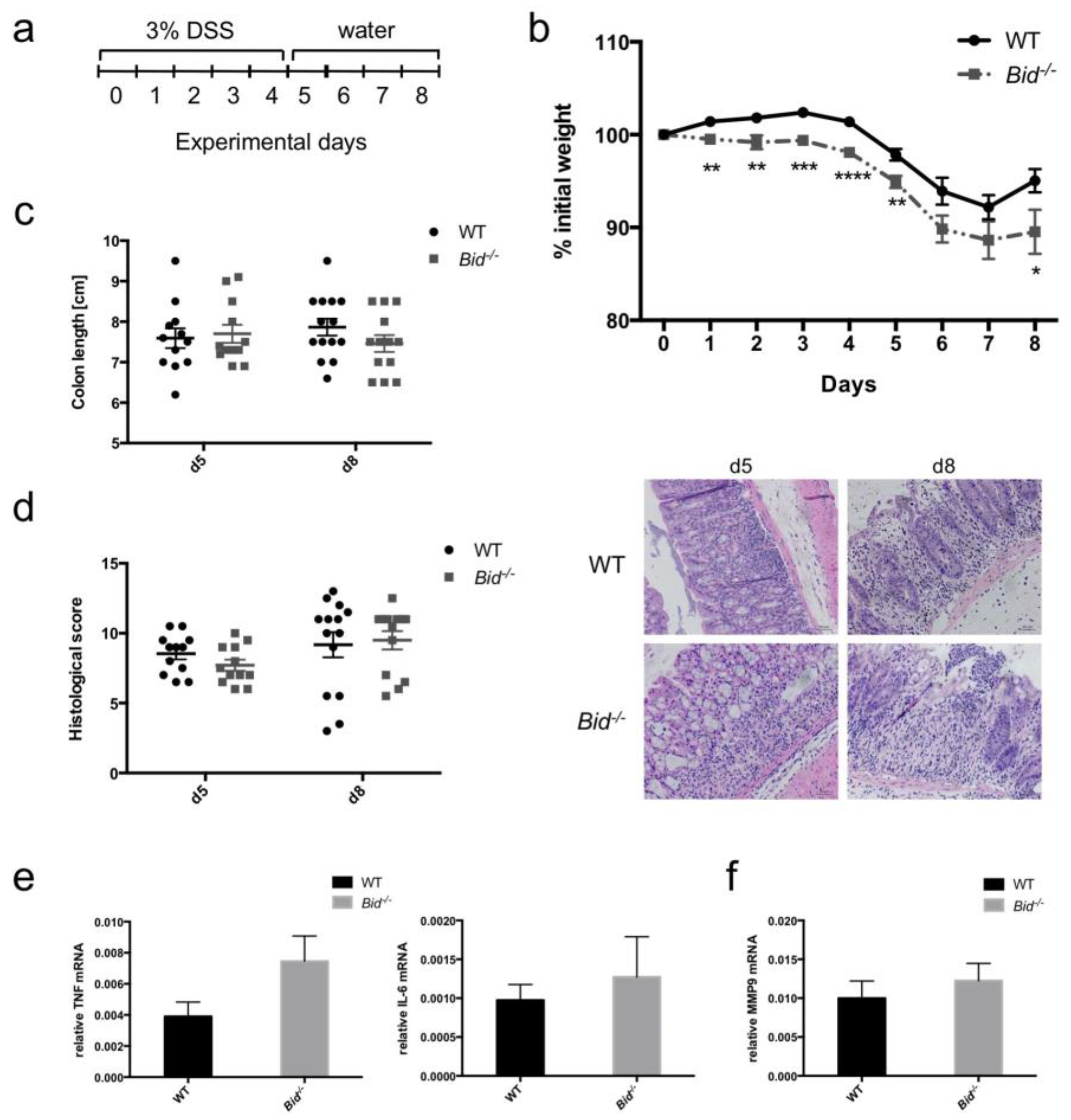
2.4. Dextran Sulfate Sodium (DSS)-Induced Weight Loss in Mice Is Aggravated in the Absence of BID
3. Discussion
4. Materials and Methods
4.1. Mice and Reagents
4.2. Isolation of Primary Neutrophils from Murine Bone Marrow
4.3. Generation and In Vitro Differentiation of Mouse Neutrophils
4.4. Gel Electrophoresis and Immunoblotting
4.5. Assessment of Cell Death by Flow Cytometry
4.6. Detection of Active Caspase-3 and -7 Activity
4.7. Fractionation by Phase Separation
4.8. DSS-Induced Colitis
4.9. Histology Scoring
4.10. qPCR Analysis
4.11. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BID | BH3-Interacting Domain Death Agonist |
| DSS | Dextran Sulfate Sodium |
| GM-CSF | Granulocyte-Macrophage colony-Stimulating Factor |
| IBD | Inflammatory Bowel Disease |
| IL-6 | Interleukin-6 |
| LPS | Lipopolysaccharide |
| Q-VD-OPh | Quinoline-Val-Asp-Difluorophenoxymethylketone |
| TNFα | Tumor Necrosis Factor Alpha |
| WT | Wild-type |
References
- Borregaard, N. Neutrophils, from marrow to microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Galani, I.E.; Andreakos, E. Neutrophils in viral infections: Current concepts and caveats. J. Leukoc. Biol. 2015, 98, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Geering, B.; Stoeckle, C.; Conus, S.; Simon, H.U. Living and dying for inflammation: Neutrophils, eosinophils, basophils. Trends Immunol. 2013, 34, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Duffin, R.; Leitch, A.E.; Fox, S.; Haslett, C.; Rossi, A.G. Targeting granulocyte apoptosis: Mechanisms, models, and therapies. Immunol. Rev. 2010, 236, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Re, F.; Polentarutti, N.; Sozzani, S.; Mantovani, A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 1992, 80, 2012–2020. [Google Scholar] [PubMed]
- Francois, S.; El Benna, J.; Dang, P.M.; Pedruzzi, E.; Gougerot-Pocidalo, M.A.; Elbim, C. Inhibition of neutrophil apoptosis by TLR agonists in whole blood: Involvement of the phosphoinositide 3-kinase/Akt and NF-κB signaling pathways, leading to increased levels of MCL-1, A1, and phosphorylated bad. J. Immunol. 2005, 174, 3633–3642. [Google Scholar] [CrossRef] [PubMed]
- Andina, N.; Conus, S.; Schneider, E.M.; Fey, M.F.; Simon, H.U. Induction of bim limits cytokine-mediated prolonged survival of neutrophils. Cell Death Differ. 2009, 16, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Villunger, A.; Scott, C.; Bouillet, P.; Strasser, A. Essential role for the BH3-only protein bim but redundant roles for BAX, BCL-2, and BCL-W in the control of granulocyte survival. Blood 2003, 101, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Kirschnek, S.; Hacker, G. Inhibition of apoptosis can be accompanied by increased bim levels in T lymphocytes and neutrophil granulocytes. Cell Death Differ. 2007, 14, 1714–1716. [Google Scholar] [CrossRef] [PubMed]
- Geering, B.; Simon, H.U. Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ. 2011, 18, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.D.; DeLeo, F.R. Neutrophil apoptosis and the resolution of infection. Immunol. Res. 2009, 43, 25–61. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.A.; Kennedy, C.L.; Pellegrini, M.; Nowell, C.J.; Zhang, J.G.; O’Reilly, L.A.; Cengia, L.; Dias, S.; Masters, S.L.; Hartland, E.L.; et al. FAS regulates neutrophil lifespan during viral and bacterial infection. J. Leukoc. Biol. 2015, 97, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Thoren, F.B.; Riise, R.E.; Ousback, J.; Della Chiesa, M.; Alsterholm, M.; Marcenaro, E.; Pesce, S.; Prato, C.; Cantoni, C.; Bylund, J.; et al. Human nk cells induce neutrophil apoptosis via an NKP46- and FAS-dependent mechanism. J. Immunol. 2012, 188, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S. FAS ligand-induced apoptosis. Annu. Rev. Genet. 1999, 33, 29–55. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.; Jost, P.J.; Nagata, S. The many roles of FAS receptor signaling in the immune system. Immunity 2009, 30, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Cullen, S.P.; Henry, C.M.; Kearney, C.J.; Logue, S.E.; Feoktistova, M.; Tynan, G.A.; Lavelle, E.C.; Leverkus, M.; Martin, S.J. FAS/CD95-induced chemokines can serve as “find-me” signals for apoptotic cells. Mol. Cell. 2013, 49, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Vercammen, D.; Brouckaert, G.; Denecker, G.; van de Craen, M.; Declercq, W.; Fiers, W.; Vandenabeele, P. Dual signaling of the FAS receptor: Initiation of both apoptotic and necrotic cell death pathways. J. Exp. Med. 1998, 188, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Holler, N.; Zaru, R.; Micheau, O.; Thome, M.; Attinger, A.; Valitutti, S.; Bodmer, J.L.; Schneider, P.; Seed, B.; Tschopp, J. FAS triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000, 1, 489–495. [Google Scholar] [CrossRef] [PubMed]
- O’ Reilly, L.; Tai, L.; Lee, L.; Kruse, E.A.; Grabow, S.; Fairlie, W.D.; Haynes, N.M.; Tarlinton, D.M.; Zhang, J.G.; Belz, G.T.; et al. Membrane-bound FAS ligand only is essential for FAS-induced apoptosis. Nature 2009, 461, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Holler, N.; Tardivel, A.; Kovacsovics-Bankowski, M.; Hertig, S.; Gaide, O.; Martinon, F.; Tinel, A.; Deperthes, D.; Calderara, S.; Schulthess, T.; et al. Two adjacent trimeric FAS ligands are required for FAS signaling and formation of a death-inducing signaling complex. Mol. Cell. Biol. 2003, 23, 1428–1440. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, H.; Xu, C.J.; Yuan, J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the FAS pathway of apoptosis. Cell 1998, 94, 491–501. [Google Scholar] [CrossRef]
- Luo, X.; Budihardjo, I.; Zou, H.; Slaughter, C.; Wang, X. BID, a BCL2 interacting protein, mediates cytochrome C release from mitochondria in response to activation of cell surface death receptors. Cell 1998, 94, 481–490. [Google Scholar] [CrossRef]
- Jost, P.J.; Grabow, S.; Gray, D.; McKenzie, M.D.; Nachbur, U.; Huang, D.C.; Bouillet, P.; Thomas, H.E.; Borner, C.; Silke, J.; et al. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature 2009, 460, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, T.; Strasser, A.; Jost, P.J. FAS death receptor signalling: Roles of BID and XIAP. Cell Death Differ. 2012, 19, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Croker, B.A.; O’Donnell, J.A.; Nowell, C.J.; Metcalf, D.; Dewson, G.; Campbell, K.J.; Rogers, K.L.; Hu, Y.; Smyth, G.K.; Zhang, J.G.; et al. FAS-mediated neutrophil apoptosis is accelerated by BID, BAK, and BAX and inhibited by BCL-2 and MCL-1. Proc. Natl. Acad. Sci. USA 2011, 108, 13135–13140. [Google Scholar] [CrossRef] [PubMed]
- Geering, B.; Gurzeler, U.; Federzoni, E.; Kaufmann, T.; Simon, H.U. A novel TNFR1-triggered apoptosis pathway mediated by class IA PI3Ks in neutrophils. Blood 2011, 117, 5953–5962. [Google Scholar] [CrossRef] [PubMed]
- Brannigan, A.E.; O’Connell, P.R.; Hurley, H.; O’Neill, A.; Brady, H.R.; Fitzpatrick, J.M.; Watson, R.W. Neutrophil apoptosis is delayed in patients with inflammatory bowel disease. Shock 2000, 13, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Perse, M.; Cerar, A. Dextran sodium sulphate colitis mouse model: Traps and tricks. J. Biomed. Biotechnol. 2012, 2012, 718617. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, H.; Huang, T.; Lei, Z.; Liu, F.; An, G.; Wen, T. Tanshinone IIa protects against dextran sulfate sodium- (DSS-) induced colitis in mice by modulation of neutrophil infiltration and activation. Oxid. Med. Cell. Longev. 2016, 2016, 7916763. [Google Scholar] [CrossRef] [PubMed]
- Sayani, F.A.; Keenan, C.M.; van Sickle, M.D.; Amundson, K.R.; Parr, E.J.; Mathison, R.D.; MacNaughton, W.K.; Braun, J.E.; Sharkey, K.A. The expression and role of FAS ligand in intestinal inflammation. Neurogastroenterol. Motil. 2004, 16, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Chen, L.; Zhang, M.; Ashton-Rickardt, P.; Turner, J.R.; Peter, M.E. CD95 is cytoprotective for intestinal epithelial cells in colitis. Inflamm. Bowel Dis. 2010, 16, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.G.; Calvo, K.R.; Pasillas, M.P.; Sykes, D.B.; Hacker, H.; Kamps, M.P. Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat. Methods 2006, 3, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Wicki, S.; Gurzeler, U.; Wei-Lynn Wong, W.; Jost, P.J.; Bachmann, D.; Kaufmann, T. Loss of XIAP facilitates switch to TNFα-induced necroptosis in mouse neutrophils. Cell Death Dis. 2016, 7, e2422. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, R.; Wicki, S.; Kaufmann, T. In vitro differentiation of mouse granulocytes. Methods Mol. Biol. 2016, 1419, 95–107. [Google Scholar] [PubMed]
- Cai, Z.; Jitkaew, S.; Zhao, J.; Chiang, H.C.; Choksi, S.; Liu, J.; Ward, Y.; Wu, L.G.; Liu, Z.G. Plasma membrane translocation of trimerized mlkl protein is required for tnf-induced necroptosis. Nat. Cell Biol. 2014, 16, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.W.; O’Neill, A.; Brannigan, A.E.; Coffey, R.; Marshall, J.C.; Brady, H.R.; Fitzpatrick, J.M. Regulation of FAS antibody induced neutrophil apoptosis is both caspase and mitochondrial dependent. FEBS Lett. 1999, 453, 67–71. [Google Scholar] [CrossRef]
- Guicciardi, M.E.; Mott, J.L.; Bronk, S.F.; Kurita, S.; Fingas, C.D.; Gores, G.J. Cellular inhibitor of apoptosis 1 (ciAP-1) degradation by caspase 8 during TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. Exp. Cell Res. 2011, 317, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, S.A.; Timmons, S.J.; Eaton, V.; Usher, L.R.; Akil, M.; Bingle, C.D.; Whyte, M.K. Inflammatory neutrophils retain susceptibility to apoptosis mediated via the FAS death receptor. J. Leukoc. Biol. 2000, 67, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Giampazolias, E.; Zunino, B.; Dhayade, S.; Bock, F.; Cloix, C.; Cao, K.; Roca, A.; Lopez, J.; Ichim, G.; Proics, E.; et al. Mitochondrial permeabilization engages NF-κB-dependent anti-tumour activity under caspase deficiency. Nat. Cell. Biol. 2017, 19, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Nachbur, U.; Vince, J.E.; O’Reilly, L.A.; Strasser, A.; Silke, J. Is bid required for nod signalling? Nature 2012, 488, E4–E6. [Google Scholar] [CrossRef] [PubMed]
- Yeretssian, G.; Correa, R.G.; Doiron, K.; Fitzgerald, P.; Dillon, C.P.; Green, D.R.; Reed, J.C.; Saleh, M. Non-apoptotic role of bid in inflammation and innate immunity. Nature 2011, 474, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, T.; Tai, L.; Ekert, P.G.; Huang, D.C.; Norris, F.; Lindemann, R.K.; Johnstone, R.W.; Dixit, V.M.; Strasser, A. The BH3-only protein bid is dispensable for DNA damage- and replicative stress-induced apoptosis or cell-cycle arrest. Cell 2007, 129, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Sun, X.; Dixit, V.M. Kinase RIP3 is dispensable for normal NF-κBs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and toll-like receptors 2 and 4. Mol. Cell Biol. 2004, 24, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Gurzeler, U.; Rabachini, T.; Dahinden, C.A.; Salmanidis, M.; Brumatti, G.; Ekert, P.G.; Echeverry, N.; Bachmann, D.; Simon, H.U.; Kaufmann, T. In vitro differentiation of near-unlimited numbers of functional mouse basophils using conditional Hoxb8. Allergy 2013, 68, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Egger, L.; Schneider, J.; Rheme, C.; Tapernoux, M.; Hacki, J.; Borner, C. Serine proteases mediate apoptosis-like cell death and phagocytosis under caspase-inhibiting conditions. Cell Death Differ. 2003, 10, 1188–1203. [Google Scholar] [CrossRef] [PubMed]
- Echeverry, N.; Bachmann, D.; Ke, F.; Strasser, A.; Simon, H.U.; Kaufmann, T. Intracellular localization of the BCL-2 family member bok and functional implications. Cell Death Differ. 2013, 20, 785–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battye, F. Flow Cytometry Consulting. Available online: http://www.frankbattye.com.au/Weasel/ (accessed on 10 December 2007).
- Wang, H.; Sun, L.; Su, L.; Rizo, J.; Liu, L.; Wang, L.F.; Wang, F.S.; Wang, X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 2014, 54, 133–146. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wicki, S.; Gurzeler, U.; Corazza, N.; Genitsch, V.; Wong, W.W.-L.; Kaufmann, T. Loss of BID Delays FASL-Induced Cell Death of Mouse Neutrophils and Aggravates DSS-Induced Weight Loss. Int. J. Mol. Sci. 2018, 19, 684. https://doi.org/10.3390/ijms19030684
Wicki S, Gurzeler U, Corazza N, Genitsch V, Wong WW-L, Kaufmann T. Loss of BID Delays FASL-Induced Cell Death of Mouse Neutrophils and Aggravates DSS-Induced Weight Loss. International Journal of Molecular Sciences. 2018; 19(3):684. https://doi.org/10.3390/ijms19030684
Chicago/Turabian StyleWicki, Simone, Ursina Gurzeler, Nadia Corazza, Vera Genitsch, Wendy Wei-Lynn Wong, and Thomas Kaufmann. 2018. "Loss of BID Delays FASL-Induced Cell Death of Mouse Neutrophils and Aggravates DSS-Induced Weight Loss" International Journal of Molecular Sciences 19, no. 3: 684. https://doi.org/10.3390/ijms19030684




