Biochemical and Physiological Characteristics of Photosynthesis in Plants of Two Calathea Species
Abstract
:1. Introduction
2. Results and Discussion
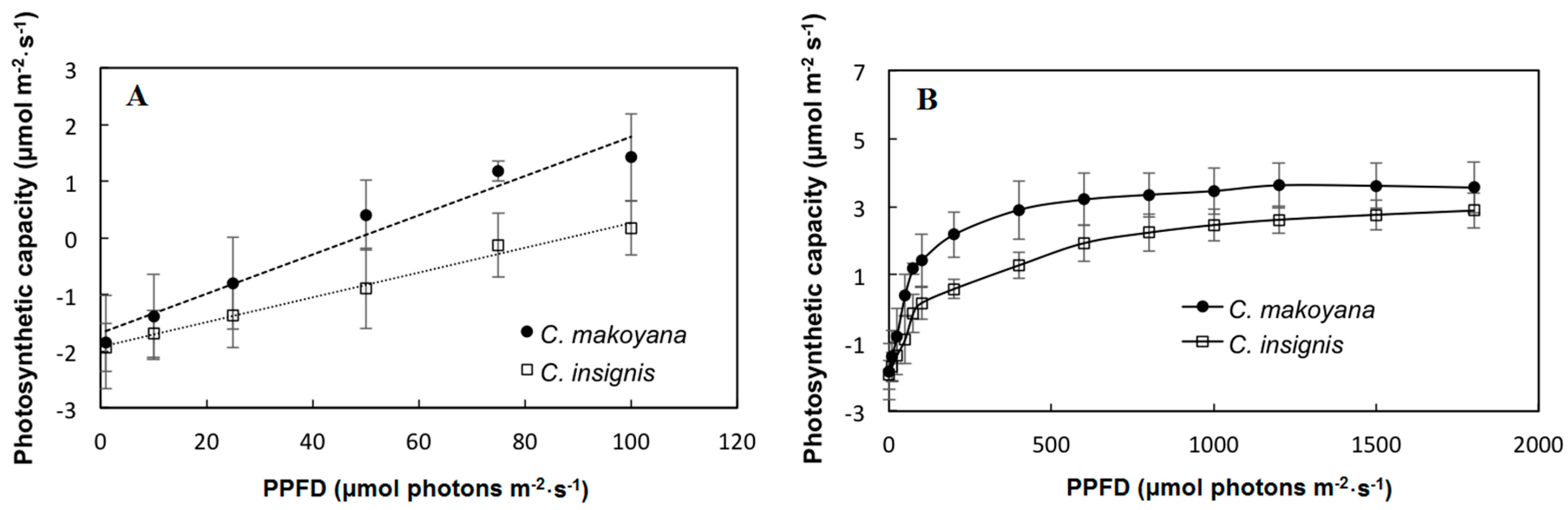
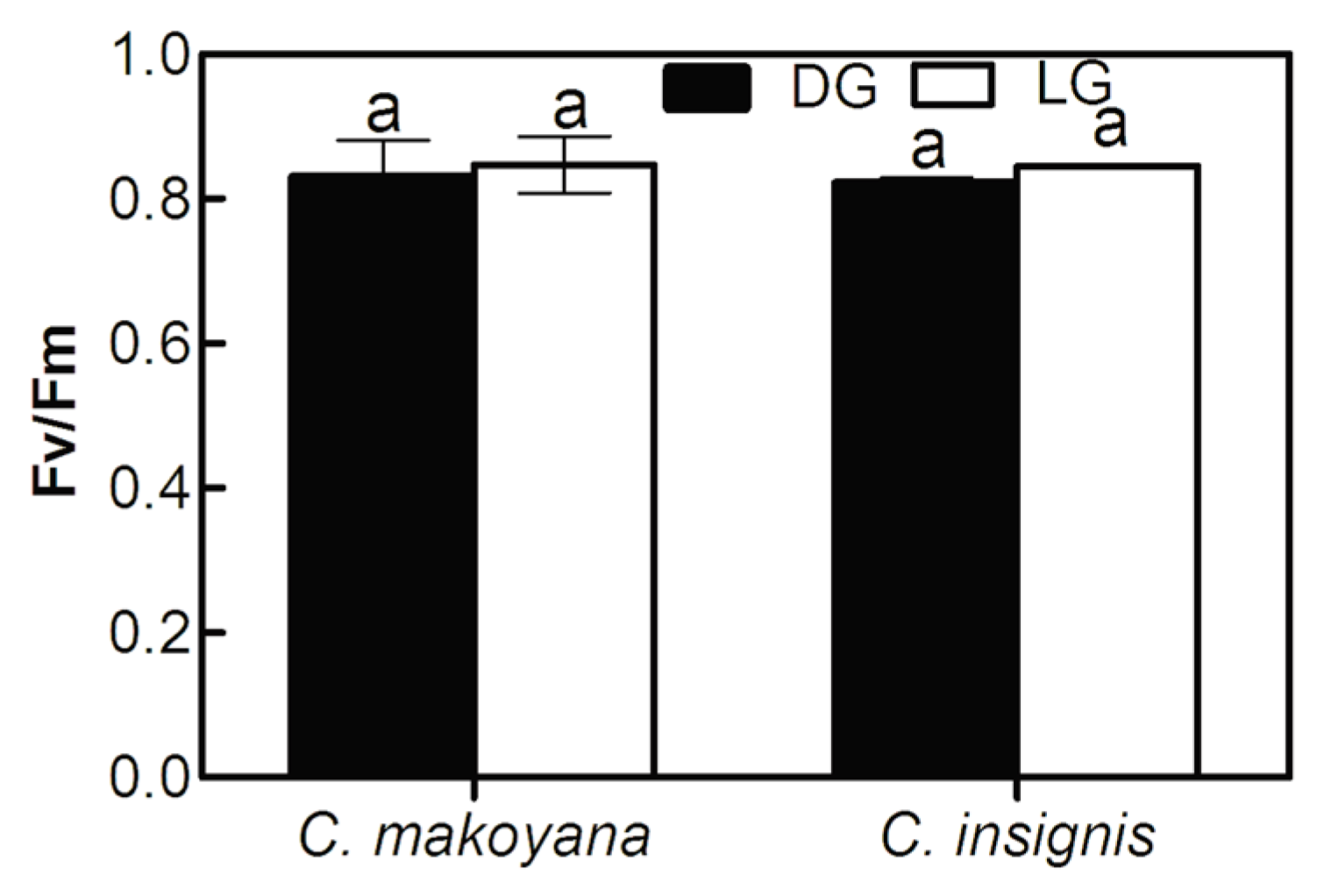
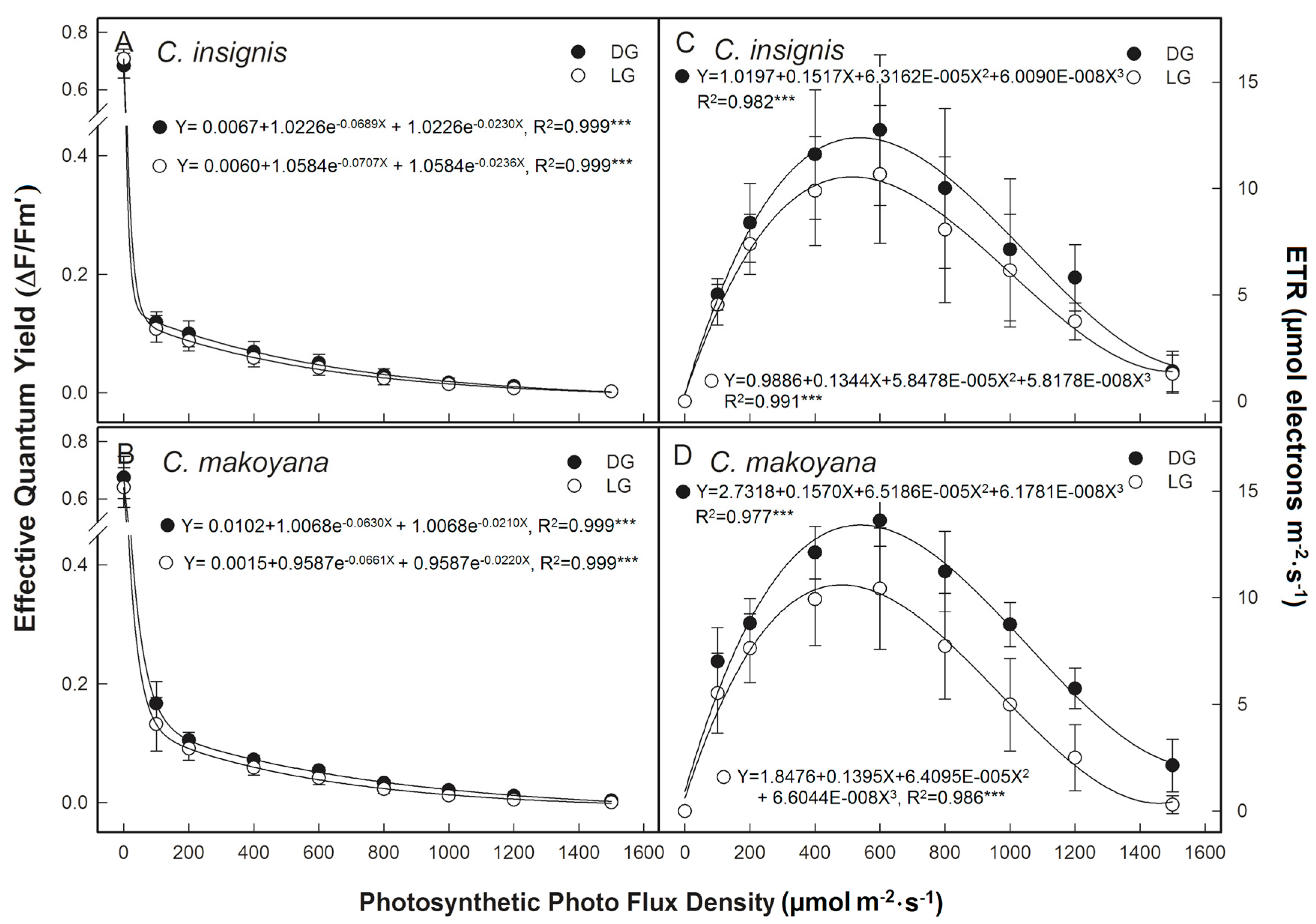
2.1. Photosynthetic Capacity and Fluorescence Parameters of C. insignis and C. makoyana
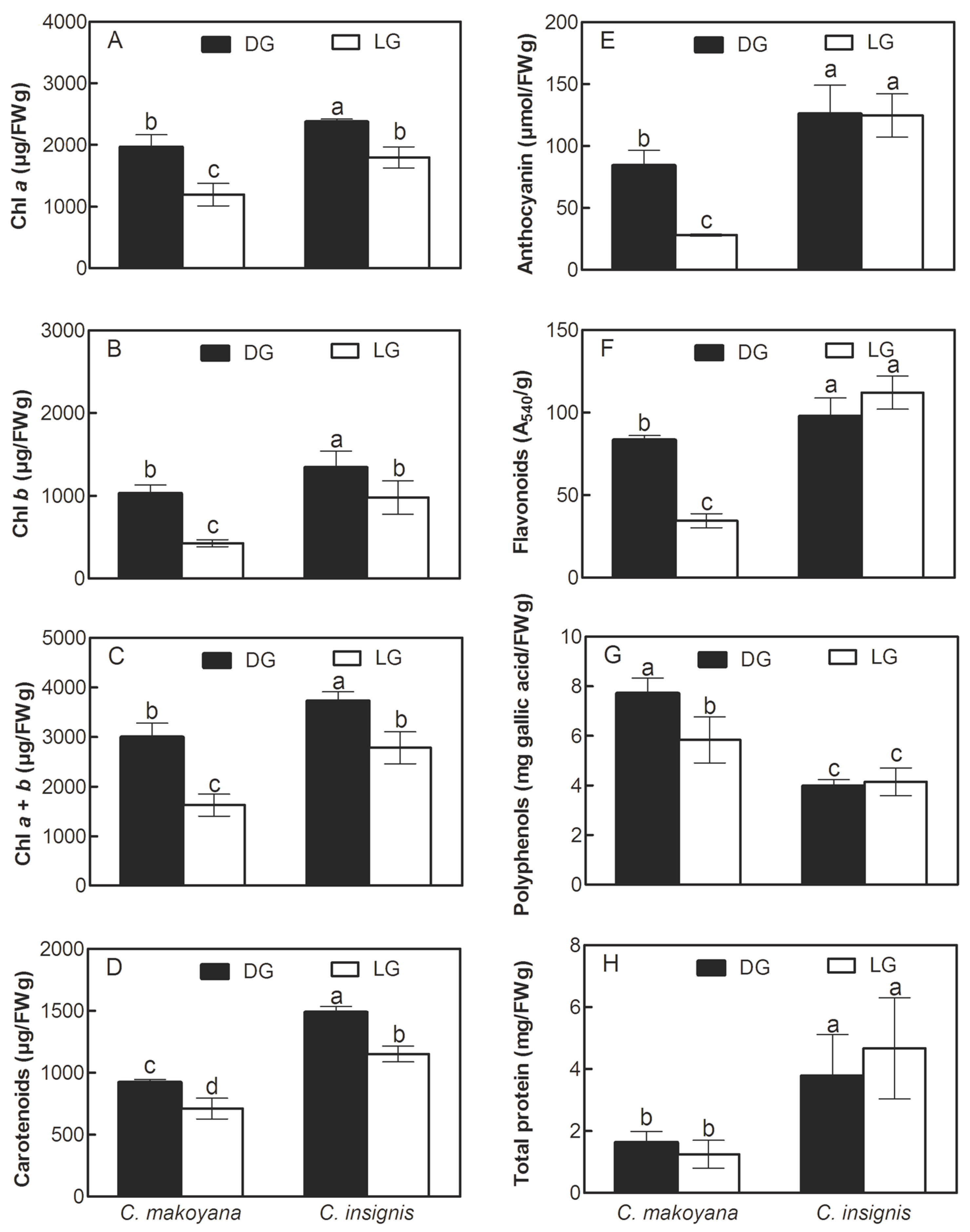
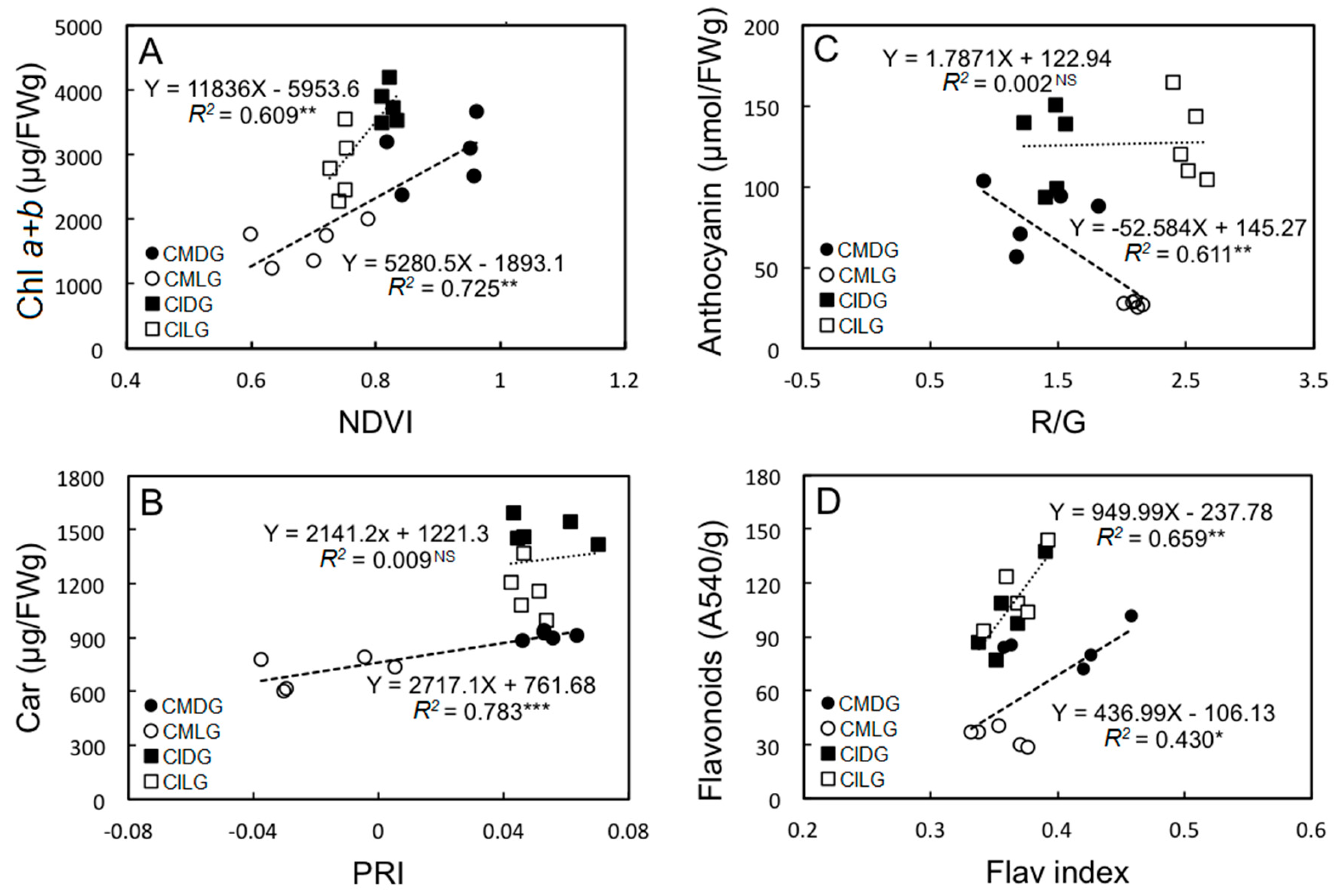
2.2. Photosynthetic Pigments, Secondary Metabolite Compounds, and Total Protein Contents
2.3. Correlations between Pigment Contents and Non-Destructive Measurements of Different Leaf Sectors
3. Materials and Methods
3.1. Plant Materials and Cultural Practice
3.2. Determination of Photosynthetic Capacity
3.3. Determination of ChlF
3.4. Determination of Chl, Car, Ant, Total Fla, PP, and Total Protein Contents
3.5. Determination of the Fla Index and Spectral Reflectance
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Chao, C.C.T.; Devanand, P.S.; Chen, J. AFLP analysis of genetic relationships among Calathea species and cultivars. Plant Sci. 2005, 168, 1459–1469. [Google Scholar] [CrossRef]
- Rout, G.R.; Senapati, S.K.; Aparajita, S. Studies on the genetic relationship among 13 cultivars of Calathea (Marantaceae) using RAPD and ISSR markers. Adv. Hortic. Sci. 2007, 21, 147–155. [Google Scholar]
- Hogewoning, S.W.; Harbinson, J. Insights on the development, kinetics, and variation of photoinhibition using chlorophyll fluorescence imaging of a chilled, variegated leaf. J. Exp. Bot. 2007, 58, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Lahiri, K.; Mukhopadhyay, M.J.; Mukhopadhyay, S. Genome analysis of species of Calathea utilizing chromosomal and nuclear DNA parameters. Nucleus 2014, 57, 203–208. [Google Scholar] [CrossRef]
- Tran, P.L.; Lee, J.S.; Park, K.H. Molecular structure and rheological character of high-amylose water caltrop (Trapa bispinosa Roxb.) starch. Food Sci. Biotechnol. 2013, 22, 979–985. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.Y.; Tian, Y.; Gong, X.Q. Composted green waste as a substitute for peat in growth media: Effects on growth and nutrition of Calathea insignis. PLoS ONE 2013, 8, e78121. [Google Scholar]
- Zhang, L.; Sun, X.Y.; Tian, Y.; Gong, X.Q. Biochar and humic acid amendments improve the quality of composted green waste as a growth medium for the ornamental plant Calathea insignis. Sci. Hortic. 2014, 176, 70–78. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Givnish, T.J. Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: Dynamic photosynthetic responses. Oecologia 2008, 155, 455. [Google Scholar] [CrossRef] [PubMed]
- Powles, S.B. Photoinhibition of photosynthesis induced by visible light. Annu. Rev. Plant Physiol. 1984, 35, 15–44. [Google Scholar] [CrossRef]
- Aleric, K.M.; Kirkman, L.K. Growth and photosynthetic responses of the federally endangered shrub, Lindera melissifolia (Lauraceae), to varied light environment. Am. J. Bot. 2005, 92, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.W.; Zarter, C.R.; Ebbert, V.; Demmig-Adams, B. Photoprotective strategies of overwintering evergreens. BioScience 2004, 54, 41–49. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W.; Barker, D.H.; Logan, B.A.; Bowlong, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Dewir, Y.H.; El-Mahrouk, M.E.S.; Al-Shmgani, H.S.; Rihan, H.Z.; Teixeira da Silva, J.A.; Fuller, M.P. Photosynthetic and biochemical characterization of in vitro-derived African violet (Saintpaulia ionantha H. Wendl) plants to ex vitro conditions. J. Plant Interact. 2015, 10, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Singsaas, E.L.; Ort, D.R.; Delucia, E.H. Diurnal regulation of photosynthesis in understory saplings. New Phytol. 2000, 145, 39–49. [Google Scholar] [CrossRef]
- Demmig-Adams, B. Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta Bioenerg. 1990, 1020, 1–24. [Google Scholar] [CrossRef]
- Anderson, J.M.; Park, Y.I.; Chow, W.S. Photoinactivation and photoprotection of photosystem II in nature. Physiol. Plant. 1997, 100, 214–223. [Google Scholar] [CrossRef]
- Molina-Bravo, R.; Arellano, C.; Sosinski, B.R.; Fernandez, G.E. A protocol to assess heat tolerance in a segregating population of raspberry using chlorophyll fluorescence. Sci. Hortic. 2011, 130, 524–530. [Google Scholar] [CrossRef]
- Banks, J.M. Continuous excitation chlorophyll fluorescence parameters: A review for practitioners. Tree Physiol. 2017, 37, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, N.; Arena, C.; De Santo, A.V. Temperature response of photosynthesis, excitation energy dissipation and alternative electron sinks to carbon assimilation in Beta vulgaris L. Environ. Exp. Bot. 2006, 55, 248–257. [Google Scholar]
- Hirotsu, N.; Makino, A.; Yokota, S.; Mae, T. The photosynthetic properties of rice leaves treated with low temperature and high irradiance. Plant Cell Physiol. 2005, 46, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Kościelniak, J.; Biesaga-Kościelniak, J. Photosynthesis and non-photochemical excitation quenching components of chlorophyll excitation in maize and field bean during chilling at different photon flux density. Photosynthetica 2006, 44, 174–180. [Google Scholar] [CrossRef]
- Melkonian, J.; Owens, T.G.; Wolfe, D.W. Gas exchange and co-regulation of photochemical and nonphotochemical quenching in bean during chilling at ambient and elevated carbon dioxide. Photosynth. Res. 2004, 79, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.L.; Chen, C.W.; Huang, H.W.; Weng, J.H. Using combined measurements of gas exchange and chlorophyll fluorescence to investigate the photosynthetic light responses of plant species adapted to different light regimes. Photosynthetica 2012, 50, 206–214. [Google Scholar] [CrossRef]
- Wong, S.L.; Huang, M.Y.; Chen, C.W.; Weng, J.H. Light induction of nonphotochemical quenching, CO2 fixation, and photoinhibition in woody and fern species adapted to different light regimes. Photosynthetica 2014, 52, 272–280. [Google Scholar] [CrossRef]
- Weng, J.H.; Chien, L.F.; Jiang, C.Y.; Shih, F.C.; Chen, H.Y. A comparison between yellow-green and green cultivars of four vegetable species in pigments, ascorbate, photosynthesis, energy dissipation, and photoinhibition. Photosynthetica 2011, 49, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Hölscher, D.; Leuschner, C.; Bohman, K.; Hagemeier, M.; Juhrbandt, J.; Tjitrosemito, S. Leaf gas exchange of trees in old growth and young secondary forest stands in Sulawesi, Indonesia. Trees 2006, 20, 278–285. [Google Scholar] [CrossRef]
- Huang, J.; Boemer, R.E.J.; Rebbeck, J. Ecophysiological responses of two herbaceous species to prescribed burning, alone or in combination with overstory thinning. Am. J. Bot. 2007, 94, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Špunda, V.; Kalina, J.; Urban, O.; Luis, V.C.; Sibisse, I.; Puertolas, J.; Šprtová, M.; Marek, M.V. Diurnal dynamics of photosynthetic parameters of Norway spruce trees cultivated under ambient and elevated CO2: The reasons of midday depression in CO2 assimilation. Plant Sci. 2005, 168, 1371–1381. [Google Scholar] [CrossRef]
- Wu, C.W.; Lin, K.H.; Lee, M.C.; Peng, Y.L.; Chou, T.Y.; Chang, Y.S. Using chlorophyll fluorescence and vegetation indices to predict the timing of nitrogen demand in Pentas lanceolata. Korean J. Hortic. Sci. Technol. 2015, 33, 845–853. [Google Scholar] [CrossRef]
- Fracheboud, Y.; Leipner, J. The application of chlorophyll fluorescence to study light, temperature, and drought stress. In Practical Applications of Chlorophyll Fluorescence in Plant Biology; DeEll, J.R., Toivonen, P.M.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 125–150. [Google Scholar]
- Müller, P.; Li, X.P.; Niyog, K.K. Non-photochemical quenching: A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Niyogi, K.K. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 2011, 155, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, G.; Johnson, G.N.; Dallosto, L.; Joliot, P.; Wollman, F.; Bassi, R. A zeaxanthinin dependent nonphotochemical quenching mechanism localized in the photosystem II core complex. Proc. Natl. Acad. Sci. USA 2004, 101, 12375–12380. [Google Scholar] [CrossRef] [PubMed]
- Kalituho, L.; Beran, K.C.; Jahns, P. The transiently generated nonphotochemical quenching of excitation energy in Arabidopsis leaves is modulated by zeaxanthin. Plant Physiol. 2007, 143, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.H.; Wong, S.L. Photosynthetic light responses of fern species adapted to different light regimes. In Ferns and Shrubs: Diversity, Cultivation and Implications for the Environment; Tuft, J., Ed.; Nova Science Publishers: New York, NY, USA, 2015; pp. 57–76. [Google Scholar]
- Zulfugarov, I.S.; Ham, O.K.; Mishra, S.R.; Kim, J.Y.; Nath, K.; Koo, H.Y.; Kim, H.S.; Moon, Y.H.; An, G.; Lee, C.H. Dependence of reaction center-type energy-dependent quenching on photosystem II antenna size. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Ebbert, V.; Mellman, D.L.; Muech, K.E.; Schaffer, L.; Funk, C.; Zarter, C.R.; Adamska, I.; Jansson, S.; Adams, W.W., III. Modulation of PsbS and flexible vs sustained energy dissipation by light environment in different species. Physiol. Plant. 2006, 127, 670–680. [Google Scholar] [CrossRef]
- Sonoike, K. Photoinhibition of photosystem I. Physiol. Plant. 2011, 142, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.; Mekala, N.R.; Aro, E.M. Photosystem II photoinhibition-repair cycle protects photosystem I from irreversible damage. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 210–215. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa-Manzano, E.; Andrade, J.L.; García-Mendoza, E.; Zotz, G.; Reyes-García, C. Photoprotection related to xanthophyll cycle pigments in epiphytic orchids acclimated at different light microenvironments in two tropical dry forests of the Yucatan Peninsula, Mexico. Planta 2015, 242, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Devitt, D.A.; Morris, R.L.; Fenstermaker, L.K. Foliar damage, spectral reflectance, and tissue ion concentrations of trees sprinkle irrigated with waters of similar salinity but different chemical composition. HortScience 2005, 40, 819–826. [Google Scholar]
- Strachan, I.B.; Pattey, E.; Boisvert, J.B. Impact of nitrogen and environmental conditions on corn as detected by hyperspectral reflectance. Remote Sens. Environ. 2002, 80, 213–224. [Google Scholar] [CrossRef]
- Bajwa, S.G.; Mishra, A.R.; Norman, R.J. Canopy reflectance response to plant nitrogen accumulation in rice. Precis. Agric. 2010, 11, 488–506. [Google Scholar] [CrossRef]
- Field, C.B.; Gamon, J.A.; Peñuelas, J. Remote sensing of terrestrialphotosynthesis. In Ecophysiology of Photosynthesis; Schulze, E.D., Caldwell, M.M., Eds.; Springer: Berlin, Germany, 1995; pp. 511–528. [Google Scholar]
- Prince, S.D. A model of regional primary production for use with coarse resolution satellite data. Int. J. Remote Sens. 1991, 12, 1313–1330. [Google Scholar] [CrossRef]
- McMichael, C.E. Estimating CO2 exchange at two sites in Arctic tundra ecosystems during the growing season using a spectral vegetation index. Int. J. Remote Sens. 1999, 20, 683–698. [Google Scholar] [CrossRef]
- Yoder, B.J.; Waring, R.H. The normalized difference vegetation index of small Douglas-fir canopies with varying chlorophyll concentrations. Remote Sens. Environ. 1994, 49, 81–91. [Google Scholar] [CrossRef]
- Gamon, J.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Nichol, C.J.; Huemmrich, K.F.; Black, T.A.; Jarvis, P.G.; Walthall, C.L.; Grace, J.; Hall, F.G. Remote sensing of photosynthetic-light-use efficiency of boreal forest. Agric. For. Meteorol. 2000, 101, 131–142. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L. and Acer platanoides L. leaves. Spectral features and relation to chlorophyll estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig-Adams, B. Regulation of photosynthetic light energy capture, conversion, and dissipation in leaves of higher plants. In Ecophysiology of Photosynthesis; Schulze, E.D., Caldwell, M.M., Eds.; Springer: Berlin, Germany, 1995; pp. 17–47. [Google Scholar]
- Frank, H.A.; Cua, A.; Chynwat, V.; Young, A.; Gosztola, D.; Wasielewski, M.R. Photophysics of the carotenoids associated with the xanthophyll cycle in photosynthesis. Photosynth. Res. 1994, 41, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Babu, T.S.; Akhtar, T.A.; Lampi, M.A.; Tripuranthakam, S.; Dixon, D.G.; Greenberg, B.M. Similar stress responses are elicited by copper and ultraviolet radiation in the aquatic plant Lemna gibba: Implication of reactive oxygen species as common signals. Plant Cell Physiol. 2003, 44, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Bidel, L.P.R.; Meyer, S.; Goulas, Y.; Cadot, Y.; Cerovic, Z.G. Responses of epidermal phenolic compounds to light acclimation: In vivo qualitative and quantitative assessment using chlorophyll fluorescence excitation spectra in leaves of three woody species. J. Photochem. Photobiol. B Biol. 2007, 88, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Kotilainen, T.; Tegelberg, R.; Julkunen-Tiitto, R.I.I.T.T.A.; Lindfors, A.; Aphalo, P.J. Metabolite specific effects of solar UV-A and UV-B on alder and birch leaf phenolics. Glob. Chang. Biol. 2008, 14, 1294–1304. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Johnson, S.L.; Liu, W.; DaSilva, N.A.; Meschwitz, S.; Dain, J.A.; Seeram, N.P. Evaluation of polyphenol anthocyanin-enriched extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry for free radical scavenging, reactive carbonyl species trapping, anti-glycation, anti-β-amyloid aggregation, and microglial neuroprotective effects. Int. J. Mol. Sci. 2018, 19, 461. [Google Scholar]
- Weng, J.H.; Jhaung, L.H.; Lin, R.J.; Chen, H.Y. Relationship between photochemical efficiency of photosystem II and the photochemical reflectance index of mango tree: Merging data from different illuminations, seasons and leaf colors. Tree Physiol. 2010, 30, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Mancinelli, A.L.; Yang, C.P.H.; Lindquist, P.; Anderson, O.; Rabino, I. Photocontrol of anthocyanin synthesis III. The action of streptomycin on the synthesis of chlorophyll and anthocyanin. Plant Physiol. 1975, 55, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Taga, M.S.; Miller, E.E.; Pratt, D.E. Chia seeds as a source of natural lipid antioxidants. J. Am. Oil Chem. Soc. 1984, 61, 928–931. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, D.; Boelman, N.T.; Turnbull, M.H.; Griffin, K.L.; Tissue, D.T.; Barbour, M.M.; Hunt, J.E.; Richardson, S.J.; Peltzer, D.A. Photosynthesis and reflectance indices for rainforest species in ecosystems undergoing progression and retrogression along a soil fertility chronosequence in New Zealand. Oecologia 2005, 144, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Stylinski, C.; Gamon, J.; Oechel, W. Seasonal patterns of reflectance indices, carotenoid pigments and photosynthesis of evergreen chaparral species. Oecologia 2002, 131, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.C.; Lin, K.-H.; Hsiung, T.-C.; Huang, M.-Y.; Yang, C.-M.; Weng, J.-H.; Hsu, M.-H.; Chen, P.-Y.; Chang, K.-C. Biochemical and Physiological Characteristics of Photosynthesis in Plants of Two Calathea Species. Int. J. Mol. Sci. 2018, 19, 704. https://doi.org/10.3390/ijms19030704
Nguyen HC, Lin K-H, Hsiung T-C, Huang M-Y, Yang C-M, Weng J-H, Hsu M-H, Chen P-Y, Chang K-C. Biochemical and Physiological Characteristics of Photosynthesis in Plants of Two Calathea Species. International Journal of Molecular Sciences. 2018; 19(3):704. https://doi.org/10.3390/ijms19030704
Chicago/Turabian StyleNguyen, Hoang Chinh, Kuan-Hung Lin, Tung-Chuan Hsiung, Meng-Yuan Huang, Chi-Ming Yang, Jen-Hsien Weng, Ming-Huang Hsu, Po-Yen Chen, and Kai-Chieh Chang. 2018. "Biochemical and Physiological Characteristics of Photosynthesis in Plants of Two Calathea Species" International Journal of Molecular Sciences 19, no. 3: 704. https://doi.org/10.3390/ijms19030704



