Effects of Passage Number and Differentiation Protocol on the Generation of Dopaminergic Neurons from Rat Bone Marrow-Derived Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Results
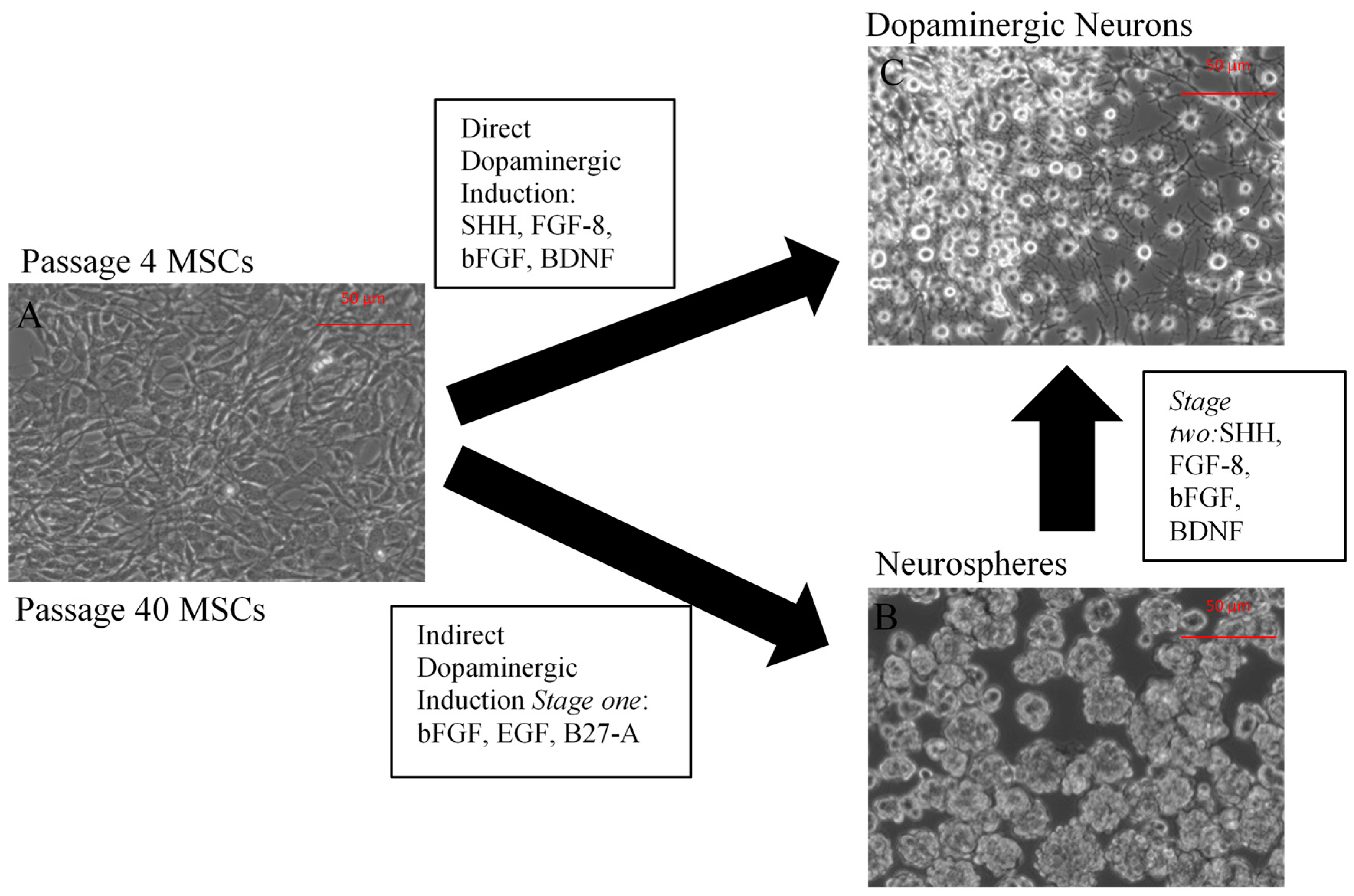
2.1. Neuronal Differentiation of Mesenchymal Stem Cells (MSCs)
2.1.1. Characterization of Undifferentiated MSCs
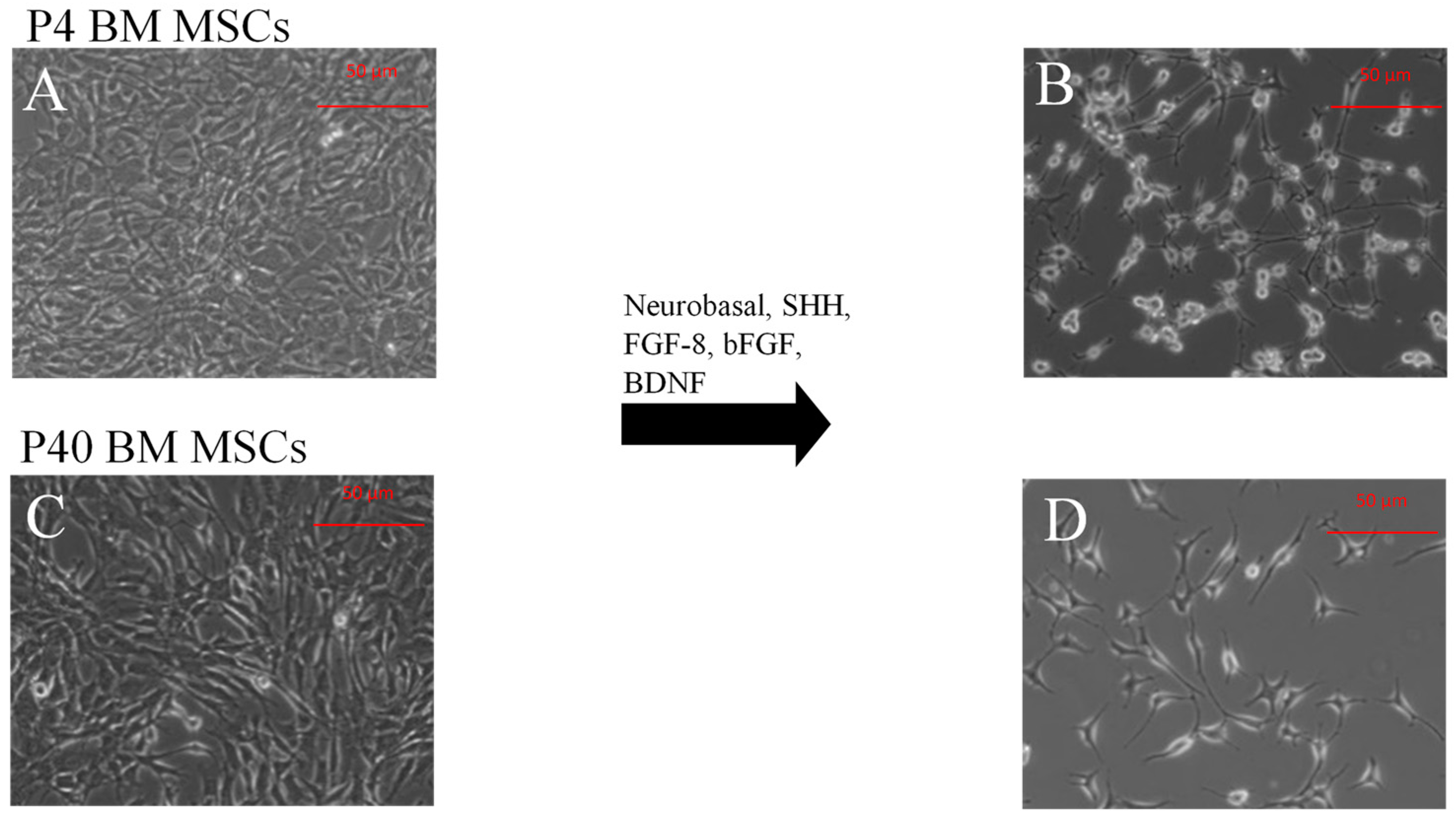
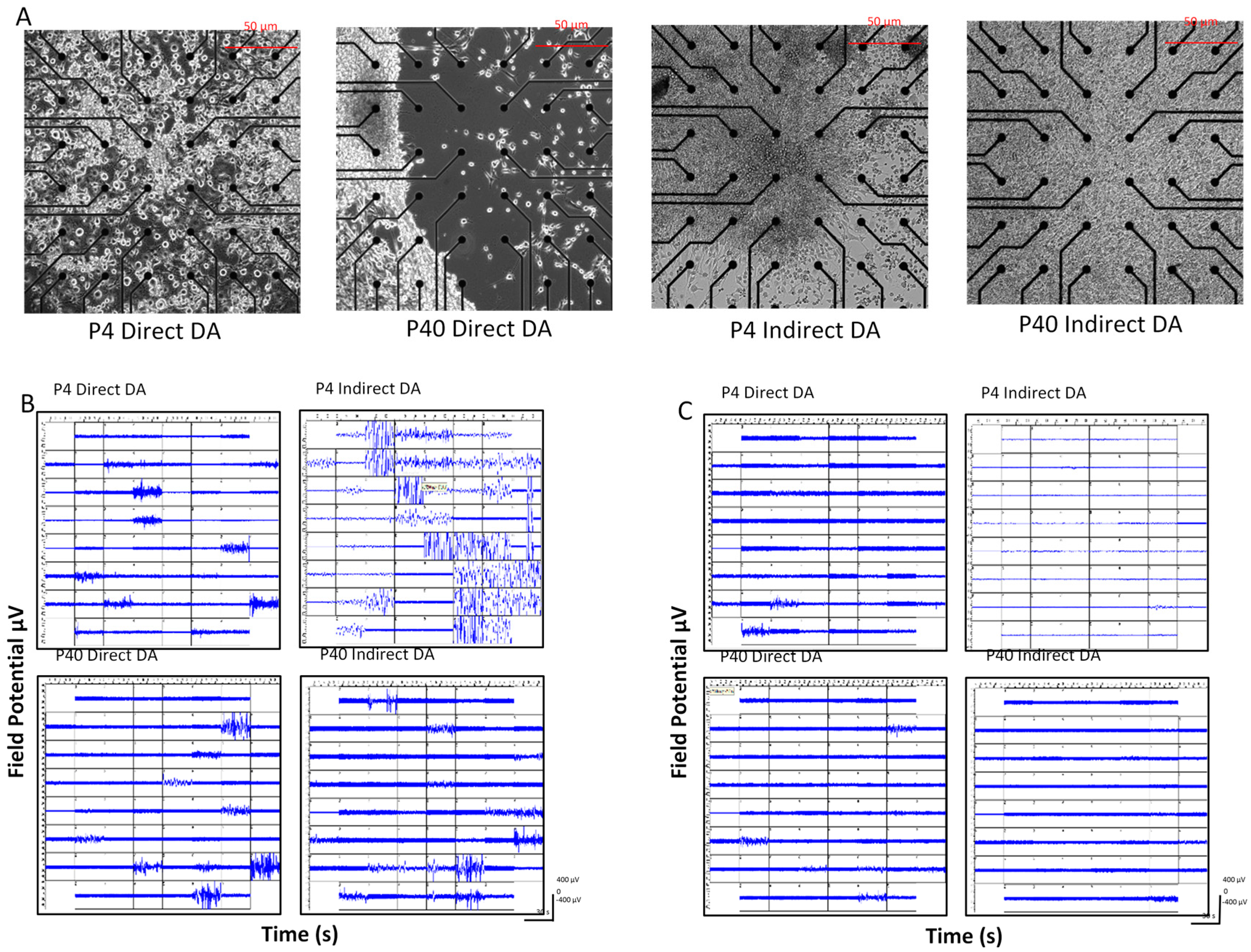
2.1.2. Direct Dopaminergic Induction
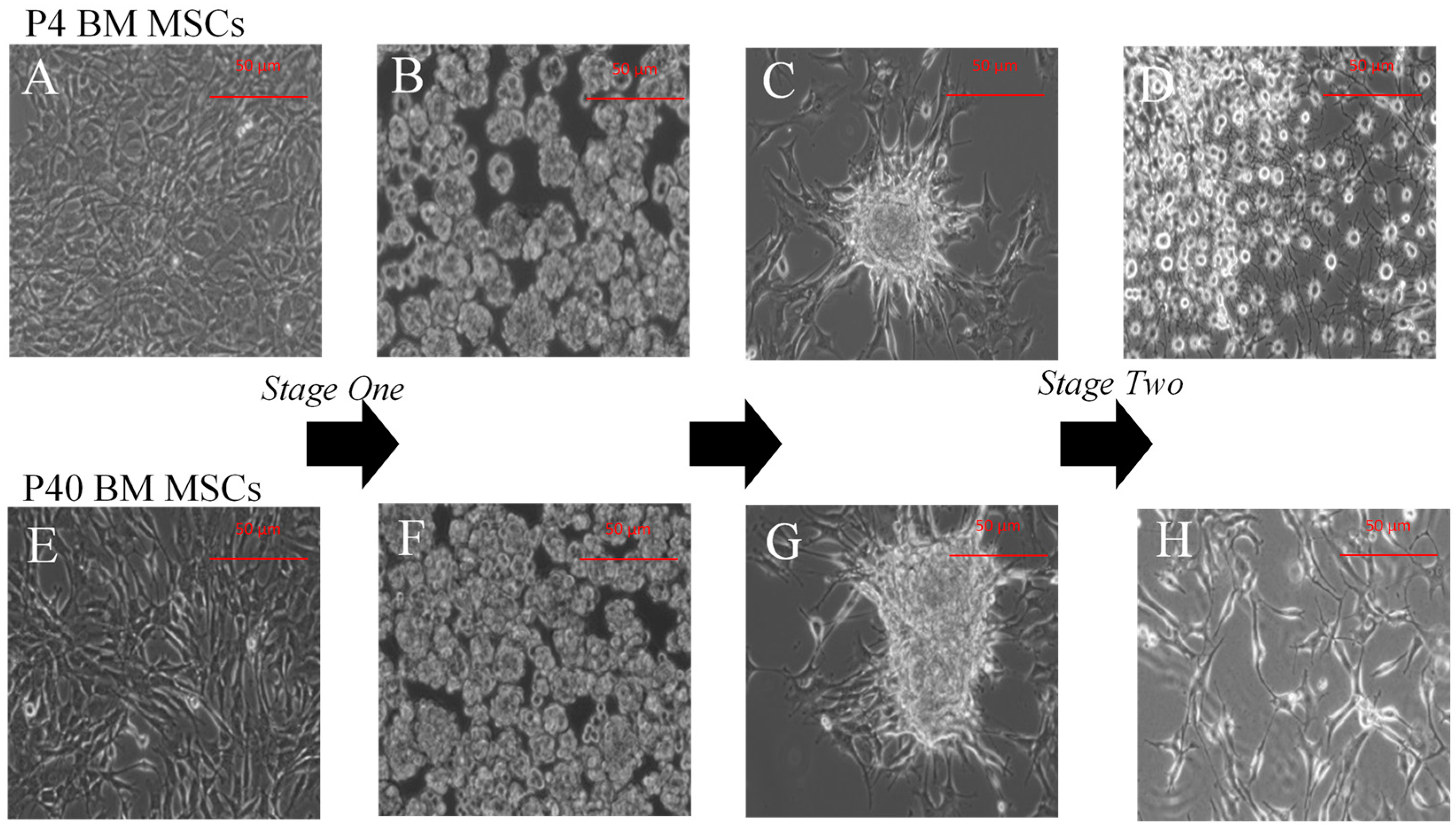
2.1.3. Indirect Dopaminergic Induction
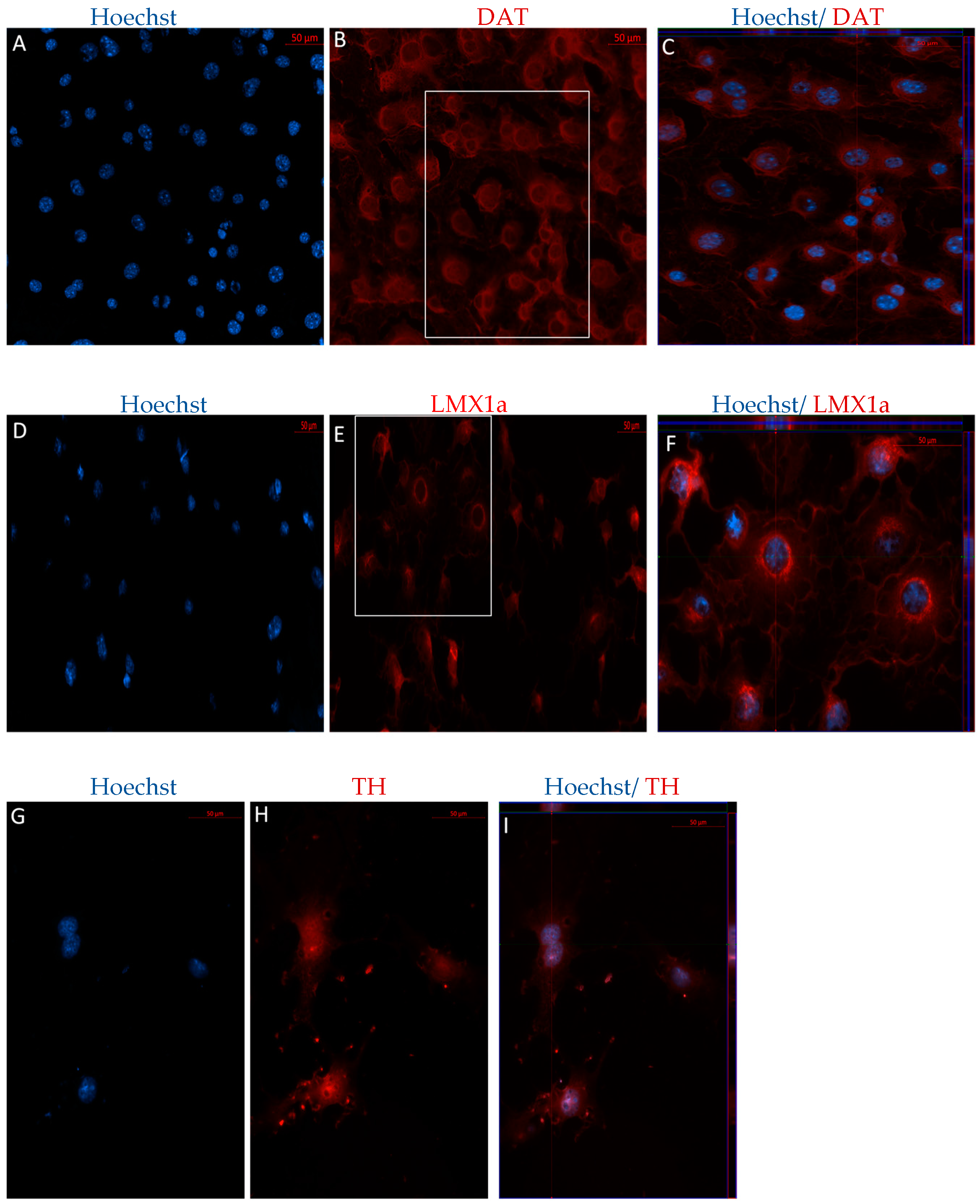
2.2. MSCs Express Neuronal and Dopaminergic-Specific Markers Following DDI and IDI
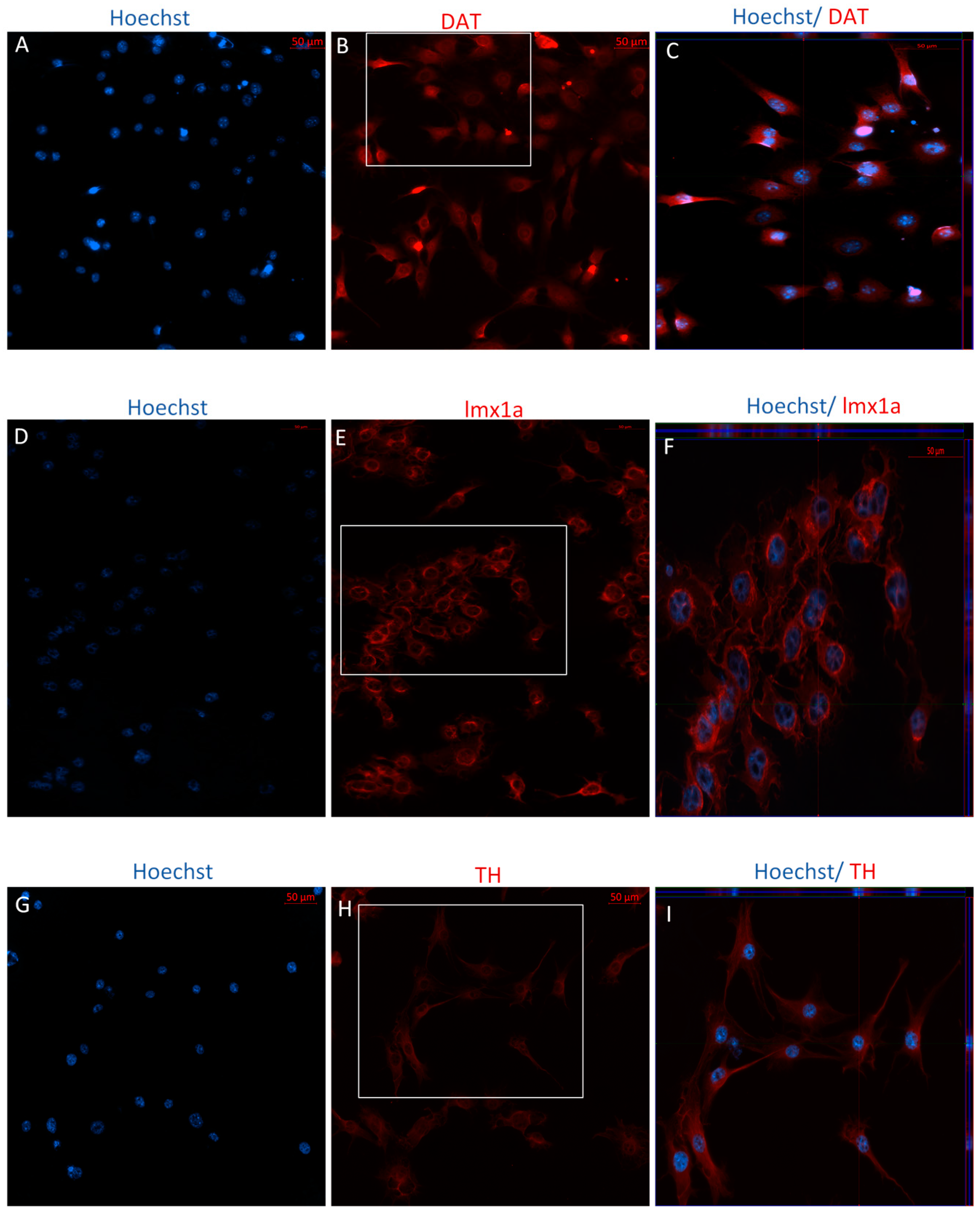
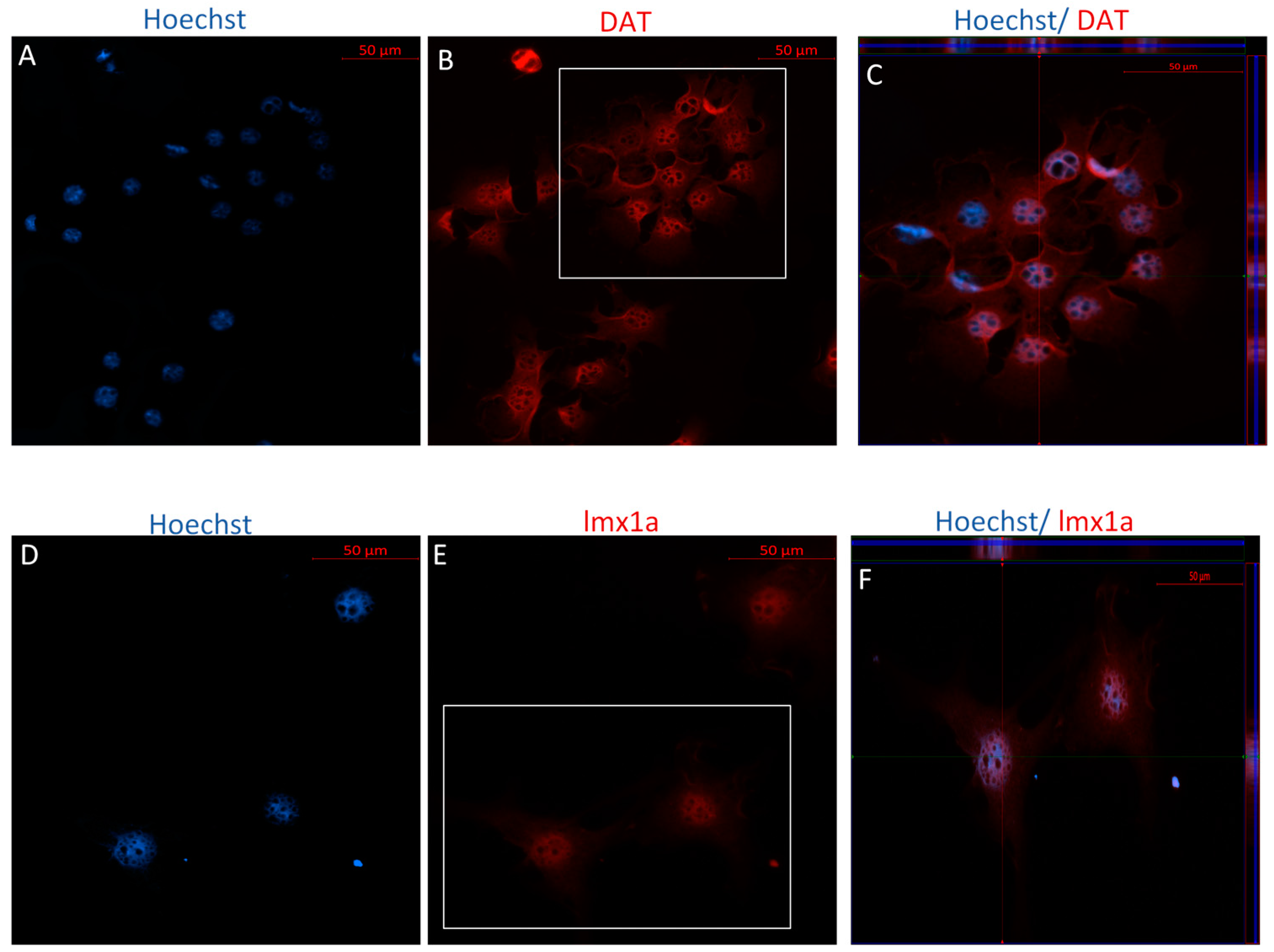
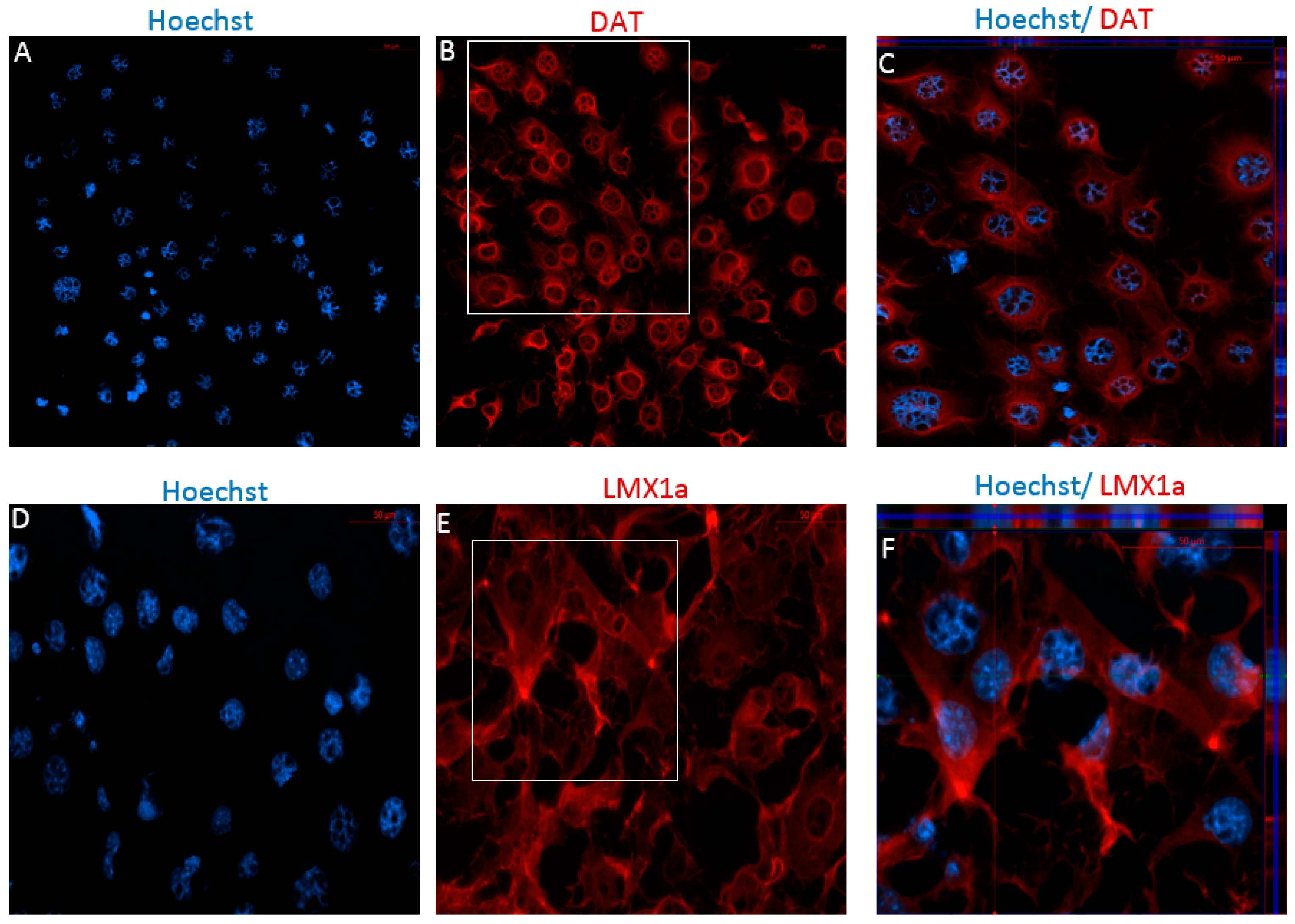
2.2.1. Immunophenotype of MSCs following the Direct Dopaminergic Induction
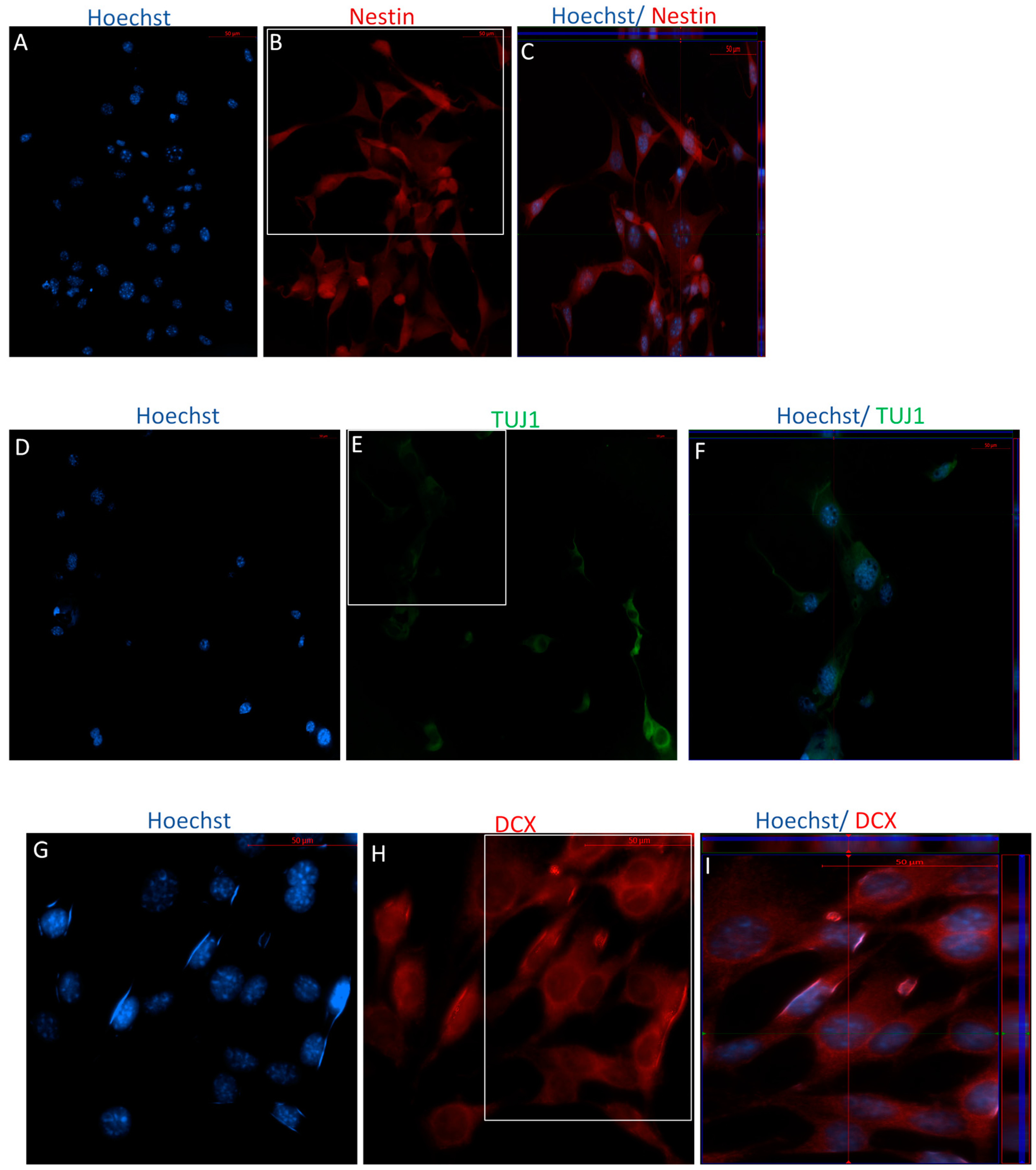
2.2.2. Immunophenotype following Stage One of the Indirect Dopaminergic Induction
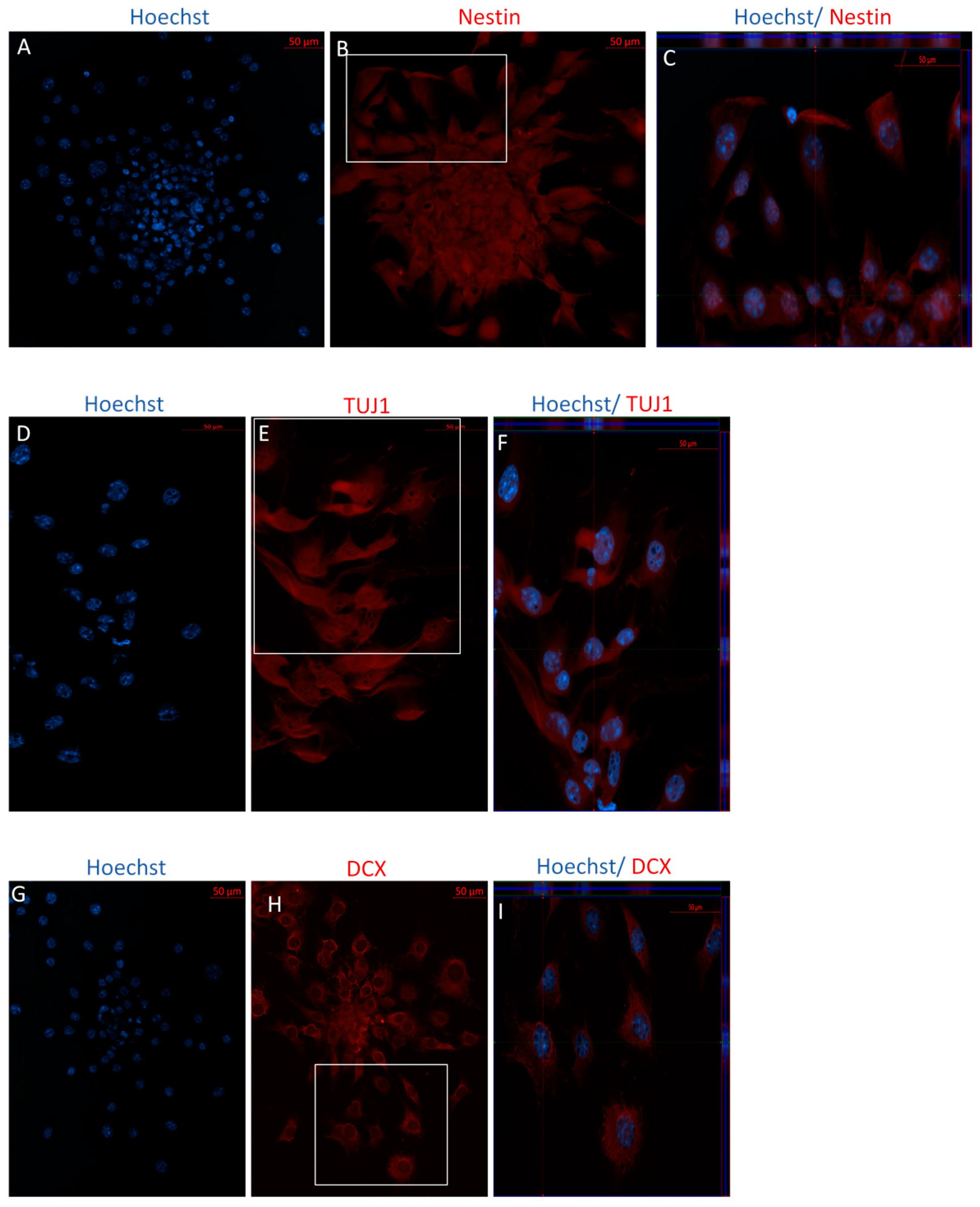
2.2.3. Immunophenotype of MSCs following Stage Two of the Indirect Dopaminergic Induction
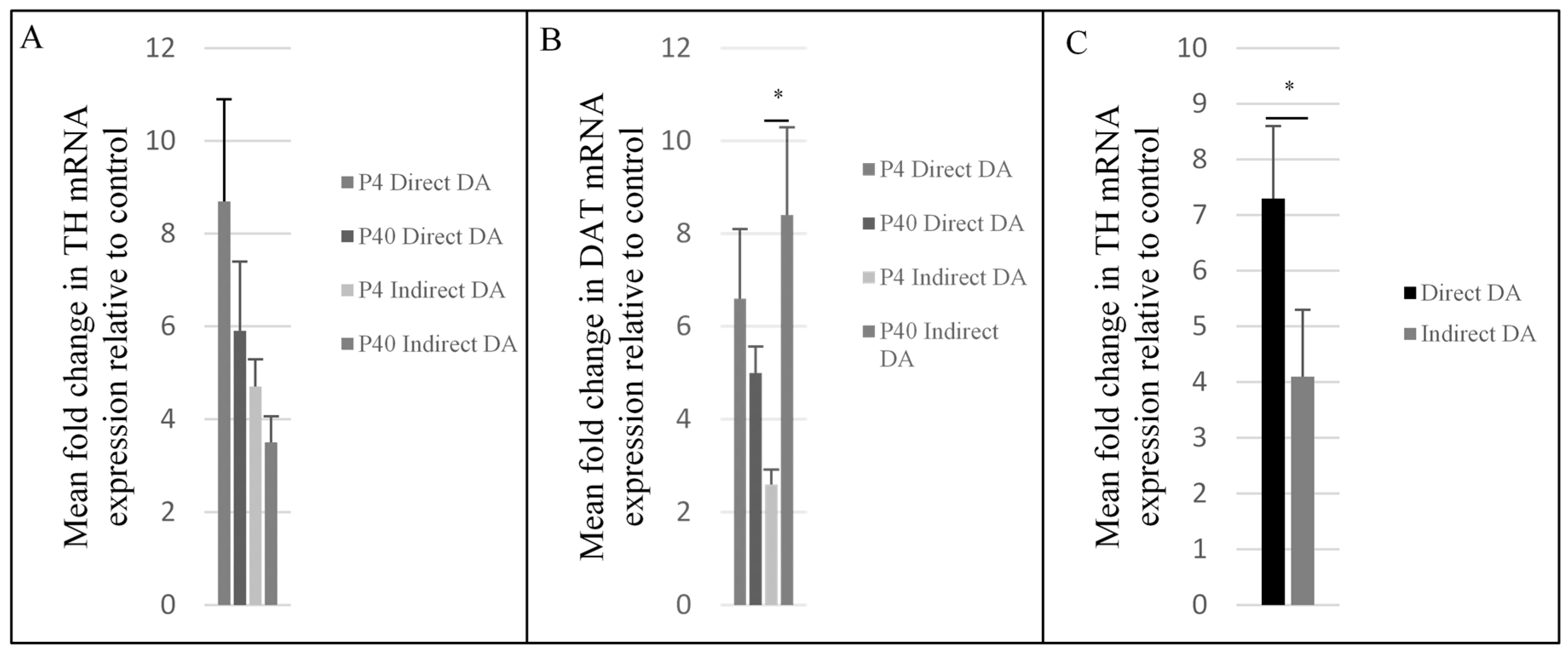
2.3. Upregulation of Dopaminergic-Specific Genes following the Direct and Indirect Dopaminergic Inductions
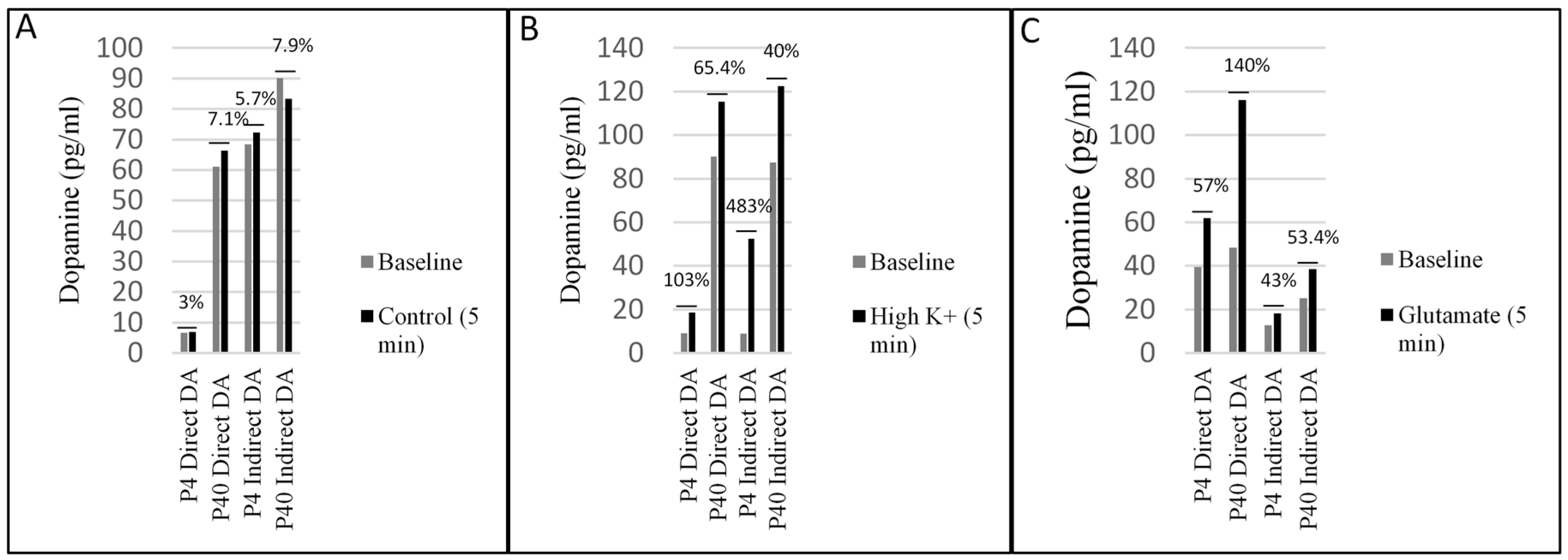
2.4. Early and Later Passaged MSCs Secrete Dopamine following the Direct and Indirect Dopaminergic Inductions
2.5. Early and Later Passaged MSCs Exhibit Electrophysiological Activity following the DDI and IDI
3. Discussion
3.1. Morphological Changes and Expression of Neuronal and Dopaminergic Markers
3.2. Effects of Passage Number on Dopaminergic Differentiation
3.3. Effects of Induction Protocol on Dopaminergic Differentiation
3.4. Co-Expression of TH and DAT
4. Materials and Methods
4.1. Isolation and Expansion of MSCs
4.2. Direct Dopaminergic Induction (DDI)
4.3. Indirect Dopaminergic Induction (IDI)
4.3.1. Stage One
4.3.2. Stage Two
4.4. Immunocytochemistry/Immunofluorescence
4.5. Flow Cytometry
4.5.1. Extracellular Staining
4.5.2. Intracellular Staining
4.5.3. Flow Cytometry Analysis
4.6. Reverse Transcriptase-Polymerase Chain Reaction
4.7. Enzyme-Linked Immunosorbent Assay
4.8. Multi-Electrode Array
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| BDNF | Brain derived neurotrophic factor |
| bFGF | Basic fibroblast growth factor |
| BHA | Butylated hydroxyanisole |
| BSA | Bovine serum albumin |
| CA | Catecholamine |
| cDNA | Complementary deoxyribonucleic acid |
| CMU | Central Michigan University |
| DA | Dopaminergic |
| DAT | Dopamine transporter |
| dcAMP | Dibutyryl cyclic AMP |
| DCX | Doublecortin |
| DDI | Direct Dopaminergic Induction |
| DMSO | Dimethyl sulfoxide |
| EGF | Epidermal growth factor |
| ELISA | Enzyme-linked immunosorbent assay |
| FACS | Flow cytometry |
| FBS | Fetal bovine serum |
| FCS | Forward scatter |
| FGF-8 | Fibroblast growth factor-8 |
| FITC | Fluorescein isothiocyanate |
| FNI | Field Neurosciences Institute |
| GAPDH | G-protein-regulated inward-rectifier potassium channel 2 |
| GDNF | Glial-derived neurotrophic factor |
| GID | Graft induced dyskinesia |
| GIRK2 | G-protein-regulated inward-rectifier potassium channel 2 |
| HBSS | Hanks balanced salt solution |
| HD | Huntington’s disease |
| HS | Horse serum |
| hUMSCs | Human umbilical cord mesenchymal stem cells |
| IACUC | Institutional Animal Care and use Committee |
| IBMX | Isobutylmethylxanthine |
| ICC | Immunocytochemistry |
| IDI | Indirect Dopaminergic Induction |
| iPSCs | Induced pluripotent stem cells |
| K+ | Potassium |
| L-DOPA | l-3,2-dihydroxyphenylalanine |
| LMX1a | LIM homeobox transcription factor 1 |
| MAP2 | Microtubule associated protein 2 |
| MEA | Mutli-electrode array |
| MCH II | Major histocompatibility complex class II |
| FNI | Field Neurosciences Institute |
| GAPDH | G-protein-regulated inward-rectifier potassium channel 2 |
| GDNF | Glial-derived neurotrophic factor |
| GID | Graft induced dyskinesia |
| GIRK2 | G-protein-regulated inward-rectifier potassium channel 2 |
| HBSS | Hanks balanced salt solution |
| HD | Huntington’s disease |
| HS | Horse serum |
| hUMSCs | Human umbilical cord mesenchymal stem cells |
| IACUC | Institutional Animal Care and use Committee |
| IBMX | Isobutylmethylxanthine |
| ICC | Immunocytochemistry |
| IDI | Indirect Dopaminergic Induction |
| iPSCs | Induced pluripotent stem cells |
| K+ | Potassium |
| l-DOPA | l-3,2-dihydroxyphenylalanine |
| LMX1a | LIM homeobox transcription factor 1 |
| MAP2 | Microtubule associated protein 2 |
| MEA | Mutli-electrode array |
| MCH II | Major histocompatibility complex class II |
| MSCs | Mesenchymal stem cells |
| NCID | Notch intracellular domain |
| NeuN | Neuronal nuclei |
| NSC | Neural stem cell |
| Nurr1 | Nuclear receptor related-1 protein |
| PBS | Phosphate buffered saline |
| PD | Parkinson’s disease |
| PDGF | Platelet-derived growth factor |
| PEI | Polyethyleneimine |
| Pitx3 | Pituitary homeobox 3 |
| qRT-PCR | Quantitative reverse transcriptase polymerase chain reaction |
| RNA | Ribonucleic acid |
| SEM | Standard error |
| SHH | Sonic hedgehog |
| SCC | Side scatter |
| TH | Tyrosine hydroxylase |
| TTX | Tetrodotoxin |
| TUJ1 | B-tubulin III |
| VTA | Ventral tegmental area |
| αMEM | Minimum essential medium alpha modification |
References
- Van Den Eeden, S.K.; Tanner, C.M.; Bernstein, A.L.; Fross, R.D.; Leimpeter, A.; Bloch, D.A.; Nelson, L.M. Incidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Farlow, J.; Pankratz, N.D.; Wojcieskzek, J.; Foroud, T. Parkinson Disease Overview. In GeneReviews [Internet]; Adam, M.P., Ardinger, H.H., Pagon, R.A., Eds.; University of Washington: Seattle, WA, USA, 2004; pp. 193–2014. [Google Scholar]
- Kuhn, D.M.; Skyes, C.E.; Geddes, T.J.; Eskow Jaunarajs, K.L.; Bishop, C. Tryptophan hydroxyls 2 aggregates through disulfide cross-linking upon oxidation: Possible link to serotonin deficits and non-motor symptoms in Parkinson’s disease. J. Neurochem. 2011, 116, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Stoessl, J.A. Etiology of Parkinson’s disease. Can. J. Neurol. Sci. 1999, 26 (Suppl. S2), S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Khoo, M.L.M.; Tao, H.; Meedeniya, A.C.B.; Mackay-Sim, A.; Ma, D.D.F. Transplantation of neuronal primed human bone marrow mesenchymal stem cells in hemiparkinsonian rodents. PLoS ONE 2011, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, F.; Drouin-Ouellet, J.; Gross, R.E. Environmental toxins and Parkinson’s disease: What have we learned from pesticide-induced animal models? Trends Pharmacol. Sci. 2009, 30, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Emre, M.; Aarsland, D.; Albanese, A.; Byrne, J.; Deuschl, G.; De Deyn, P.P.; Durif, F.; Kulisevsky, J.; van Laar, T.; Lees, A.; et al. Rivastigmine for dementia associated with Parkinson’s disease. N. Engl. J. Med. 2004, 351, 2509–2518. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Chinea, P.; Bezard, E. Basal ganglia circuits underlying the pathophysiology of levodopa-induced dyskinesia. Front. Neuroanat. 2010, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Eskow Jaunarajs, K.L.; Angoa-Perez, M.; Kuhn, D.M.; Bishop, C. Potential mechanisms underlying anxiety and depression in Parkinson’s disease: Consequences of L-DOPA treatment. Neurosci. Biobehav. Rev. 2011, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, E.; Koh, S.E.; Maeng, S.; Lee, W.D.; Lim, J.; Shim, I.; Lee, Y.J. Dopaminergic differentiation of neural progenitors derived from placental mesenchymal stem cells in the brains of Parkinson’s disease model rats and alleviation of asymmetric rotational behavior. Brain Res. 2012, 1466, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Danes, A.; Richaud-Patin, Y.; Carballo-Carbajal, I.; Jimenez-Delgado, S.; Caig, C.; Mora, S.; Guglielmo, C.D.; Ezauerra, M.; Patel, B.; Giralt, A.; et al. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol. Med. 2012, 4, 380–395l. [Google Scholar] [CrossRef] [PubMed]
- Shapira, A.H.V. Etiology of Parkinson’s disease. Neurology 2006, 66, S10–S23. [Google Scholar] [CrossRef]
- Datta, I.; Bhonde, R. Can mesenchymal stem cells reduce vulnerability of dopaminergic neurons in the substantia nigra to oxidative insult in individuals at risk to Parkinson’s disease? Cell Biol. Int. 2012, 36, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Brundin, P.; Widner, H.; Rehncrona, S.; Gustavii, B.; Frackowiak, R.; Leenders, K.L.; Sawle, G.; Rothwell, J.C.; Marsden, C.D. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science 1990, 247, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Freeman, T.B.; Snow, B.J.; Francois, J.G.; Vingerhoets, M.D.; Mufson, E.J.; Sanberg, P.R.; Hauser, R.A.; Smith, D.A.; Nauert, M.; et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N. Engl. J. Med. 1995, 332, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Rosenstein, J.M.; Collier, T.J.; Burke, M.A.; Chen, E.Y.; Li, J.M.; Martel, L.; Levey, A.E.; Mufson, E.J.; Freeman, T.B.; et al. Functional fetal nigral grafts in a patient with Parkinson’s disease: Chemoanatomic, ultrastructural, and metabolic studies. J. Comp. Neurol. 1996, 370, 203–230. [Google Scholar] [CrossRef]
- Wenning, G.K.; Odin, P.; Morrish, P.; Rehncrona, S.; Widner, H.; Brundin, P.; Rothwell, J.C.; Brown, R.; Gustavii, B.; Hagell, P.; et al. Short- and long-term survival and function of unilateral instrastriatal dopaminergic grafts in Parkinson’s disease. Ann. Neurol. 1997, 42, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Piccini, P.; Brooks, D.J.; Bjorklund, A.; Gunn, R.N.; Grasby, P.M.; Rimoldi, O.; Brundin, P.; Hagell, P.; Rehncrona, S.; Widner, H.; et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat. Neurosci. 1999, 2, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Brundin, P.; Pogarell, O.; Hagell, P.; Piccini, P.; Widner, H.; Schrag, A.; Kupsch, A.; Crabb, L.; Odin, P.; Gustabii, B.; et al. Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson’s disease. Brain 2000, 123, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Freed, C.R.; Greene, P.E.; Breeze, R.E.; Tsai, W.; DuMouchel, W.; Kao, R.; Dillon, S.; Winfield, H.; Culver, S.; Trojanowski, J.Q.; et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med. 2001, 344, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Bjorklund, A. Cell therapy in Parkinson’s disease. NeuroRx 2004, 1, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yang, M.; Poremsky, E.; Kidd, S.; Schneider, J.S.; Iacovitti, L. Dopaminergic neurons derived from human induced pluripotent stem cells survive and integrate into 6-OHDA-lesioned rats. Stem Cell Dev. 2010, 19, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, Y.; Zhang, S.C. Directed differentiation of dopamine neurons from human pluripotent stem cells. Methods Mol. Biol. 2011, 767, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Theka, I.; Caiazzo, M.; Dvoretskova, E.; Leo, D.; Ungaro, F.; Curreli, S.; Manago, F.; Dell’Anno, M.T.; Pezzoli, G.; Gainetdinov, R.R.; et al. Rapid generation of functional dopaminergic neurons from human induced pluripotent stem cells through a single-step procedure using cell lineage transcription factors. Stem Cells Transl. Med. 2013, 2, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Greene, P.E.; Fahn, S.; Tsai, W.Y. Severe spontaneous dyskinesias: A disabling complication of embryonic dopaminergic tissue implants in a subset of transplanted patients with advance Parkinson’s disease. Mov. Disord. 1999, 14, 904. [Google Scholar]
- Hagell, P.; Piccini, P.; Bjorklund, A.; Brundin, P.; Rehncrona, S.; Widner, H.; Crabb, L.; Pavese, N.; Oertrel, W.H.; Quinn, N.; et al. Dyskinesias following neural transplantation in Parkinson’s disease. Nat. Neurosci. 2002, 5, 627–628. [Google Scholar] [CrossRef] [PubMed]
- Brundin, P.; Barker, R.A.; Parmar, M. Neural grafting in Parkinson’s disease problems and possibilities. Prog. Brain Res. 2010, 184, 265–294. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 2007, 448, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, D.; Maehr, R.; Guo, W.; Eijkelenboom, A.; Snitow, M.; Chen, A.E.; Melton, D.A. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol. 2008, 26, 795–797. [Google Scholar] [CrossRef] [PubMed]
- Wernig, M.; Meissner, A.; Cassady, J.P.; Jaenisch, R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell 2008, 2, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Freed, C.R. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells 2009, 27, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Yamamoto, H.; Miyoshi, N.; Takahashi, H.; Suzuki, Y.; Haraguchi, N.; Ishii, H.; Doki, Y.; Mori, M. Emerging methods for preparing iPS cells. Jpn. J. Clin. Oncol. 2012, 42, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Fink, K.D.; Rossignol, J.; Lu, M.; Leveque, X.; Hulse, T.D.; Crane, A.T.; Nerrier-Daquin, V.; Wyse, R.D.; Starski, P.A.; Schloop, M.T.; et al. Survival and differentiation of adenovirus-generated induced pluripotent stem cells transplanted into the rat striatum. Cell Transplant. 2014, 23, 1407–1423. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A. Mesenchymal stem-cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Minguell, J.J.; Erices, A.; Conget, P. Mesenchymal stem cells. Exp. Biol. Med. 2001, 226, 507–520. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005, 2. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Song, S.U. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch. Pharm. Res. 2012, 35, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.; Annett, G.; Wirthlin, L.; Olson, S.; Bauer, G.; Nolta, J.A. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regener. Med. 2010, 5, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Chern, Y.; Shen, C.K.J.; Wen, H.L.; Chang, Y.C.; Li, H.; Cheng, T.H.; Hsieh-Li, H.M. Human mesenchymal stem cells prolong survival and ameliorate motor deficit through trophic support in Huntington’s disease mouse models. PLoS ONE 2011, 6, e22924. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ramos, J.; Song, S.; Cardozo-Pelaez, F.; Hazzi, C.; Stedeford, T.; Willing, A.; Freeman, T.B.; Saporta, S.; Janssen, W.; Patel, N.; et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 2000, 164, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Obrocka, M.; Fischer, I.; Prockop, D.J. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracelluar cyclic AMP. Biochem. Biophys. Res. Commun. 2001, 282, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Hermann, A.; Gastl, R.; Liebau, S.; Popa, M.O.; Fielder, J.; Boehm, B.O.; Maisel, M.; Lerche, H.; Schwarz, J.; Brenner, R.; et al. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J. Cell Sci. 2004, 117, 4411–4422. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhu, L.; Huang, Y.; Lee, T.D.; Forman, S.J.; Shih, C.C. Derivation of neural stem cells from mesenchymal stem cells: Evidence for a biopotential stem cell population. Stem Cells Dev. 2008, 17, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Cui, Y.C.; Wang, X.J.; Wu, A.L.; Hu, G.F.; Luo, F.L.; Sun, J.K.; Sun, J.; Wu, L.K. Neural progenitor cells derived from adult bone marrow mesenchymal stem cells promote neuronal growth. Life Sci. 2012, 91, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Black, I.B.; Woodbury, D. Adult rat and human bone marrow stromal stem cells differentiate into neurons. Blood Cells Mol. Dis. 2001, 27, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Dezawa, M.; Kanno, H.; Hoshino, M.; Cho, H.; Matsumoto, N.; Itokazu, Y.; Tajima, N.; Yamada, H.; Sawada, H.; Ishikawa, H.; et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J. Clin. Investig. 2004, 113, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Olszewski, M.; Huang, W.; Kletzel, M. Neural cell differentiation in vitro from adult human bone marrow mesenchymal stem cells. Stem Cells Dev. 2005, 14, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Peterson, E.B.; Steindler, D.A.; Jorgenesn, M.L.; Laywell, E.D. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells 2006, 14, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Scintu, F.; Reali, C.; Pillai, R.; Badiali, M.; Sanna, M.A.; Argiolu, F.; Ristaldi, M.S.; Sogos, V. Differentiation of human bone marrow stem cells into cells with a neural phenotype: Diverse effects of two specific treatments. BMC Neurosci. 2006, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Seitz, L.C.; Abramcyzk, A.M.; Liu, L.; Chan, C. cAMP initiates early phase neuron-like morphology changes and late phase neural differentiation in mesenchymal stem cells. Cell. Mol. Life Sci. 2011, 68, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Divya, M.S.; Roshin, G.E.; Divya, T.S.; Rasheed, V.A.; Santhoshkumar, T.R.; Elizabeth, K.E.; James, J.; Pillai, R.M. Umbilical cord blood-derived mesenchymal stem cells consist of a unique population of progenitors co-expressing mesenchymal stem cell and neuronal marker capable of instantaneous neuronal differentiation. Stem Cell Res. Ther. 2012, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yin, F.; Meng, H.Q.; Ling, L.; Hu-He, T.N.; Li, P.; Zhang, C.X.; Yu, S.; Duan, D.S.; Fan, H.X. Differentiation of mesenchymal stem cells into dopaminergic neuron-like cells in vitro. Biomed. Environ. Sci. 2005, 18, 36–42. [Google Scholar] [PubMed]
- Fu, Y.S.; Cheng, Y.C.; Lin, M.Y.A.; Cheng, H.; Chu, P.M.; Chou, S.C.; Shih, Y.H.; Ko, M.H.; Sung, M.S. Conversion of human umbilical cord mesenchymal stem cells in wharton’s jelly to dopaminergic neurons in vitro: Potential therapeutic application for parkinsonism. Stem Cells 2006, 24, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Suon, S.; Yang, M.; Iacovitti, L. Adult human bone marrow stromal spheres express neuronal traits in vitro and in a rat model of Parkinson’s disease. Brain Res. 2006, 1106, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Trzaska, K.A.; Kuzhikandathil, E.V.; Rameshwar, P. Specification of a dopaminergic phenotype from adult human mesenchymal stem cells. Stem Cells 2007, 25, 2797–2808. [Google Scholar] [CrossRef] [PubMed]
- Barzilay, R.; Kan, I.; Ben-Zur, T.; Bulvik, S.; Melamed, E.; Offen, D. Induction of human mesenchymal stem cells into dopamine-producing cells with different differentiation protocols. Stem Cells Dev. 2008, 17, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Tio, M.; Tan, K.H.; Lee, W.; Wang, T.T.; Udolph, G. Roles of db-cAMP, IBMX and RA in aspects of neural differentiation of cord blood derived mesenchymal-like stem cells. PLoS ONE 2010, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Trzaska, K.A.; Rameshwar, P. Dopaminergic neuronal differentiation protocol for human mesenchymal stem cells. Methods Mol. Biol. 2011, 698, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Lv, C.; Zhuang, W.; Chen, D.; Lv, E.; Li, F.; Wang, X. An effective inducer of dopaminergic neuron-like differentiation. Neural Regener. Res. 2013, 8, 427–434. [Google Scholar] [CrossRef]
- Baxter, M.; Wynn, R.F.; Jowitt, S.N.; Wraith, J.E.; Fairbairn, L.J.; Bellantuono, I. Study of telomere length reveal rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 2004, 22, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Bonab, M.M.; Alimoghaddam, K.; Talebain, F.; Ghaffari, S.H.; Ghavamzadeh, A.; Nikbin, B. Aging of Mesenchymal stem cell in vitro. BMC Cell Biol. 2006, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Khoo, M.L.M.; Shen, B.; Tao, H.; Ma, D.D. Long-term serial passage and neuronal differentiation capability of human bone marrow mesenchymal stem cells. Stem Cells Dev. 2008, 17, 883–896. [Google Scholar] [CrossRef] [PubMed]
- DiGirolamo, C.M.; Stokes, D.; Colter, D.; Phinney, D.G.; Class, R.; Prockop, D.J. Propagation and senescence of human marrow stromal cells in culture: A simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br. J. Haematol. 1999, 107, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Stenderup, K.; Justesen, J.; Clausen, C.; Kassem, M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 2003, 33, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, V.; Kong, E.; Suzuki, G.; Sato, K.; Canty, J.M.; Lee, T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J. Cell. Physiol. 2005, 205, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Horn, P.; Castoldi, M.; Diehlmann, A.; Bork, S.; Saffrich, R.; Benes, V.; Blake, J.; Pfiser, S.; Ecstein, V.; et al. Replicative senescence of mesenchymal stem cells: A continuous and organized process. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.; Hong, S.; Kang, J.; Woo, J.; Park, J.; Lee, J.; Seo, J.S. Identification of senescence-associated genes in human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2008, 371, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Alexanian, A.R. The neural plasticity of early-passage human bone marrow-derived mesenchymal stem cells and their modulation with chromatin-modifying agents. J. Tissue Eng. Regen. Med. 2012, 8, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Peperzak, V.; van Rijn, L.; Borst, J.; de Bruijn, J.D. Dexamethasone treatment during the expansion phase maintains stemness of bone marrow mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2010, 4, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Blesch, A.; Tuszynski, M.H. Induction of bone marrow stromal cells to neurons: Differentiation, transdifferentiation, or artifact? J. Neurosci. Res. 2004, 77, 174–191. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.S.; Bahat-Stroomza, M.; Barzilay, R.; Burshtein, A.; Bulvik, S.; Barhum, Y.; Panet, H.; Melamed, E.; Offen, D. Regenerative effect of neural-induced human mesenchymal stromal cells in rat models of Parkinson’s disease. Cytotherapy 2008, 10, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, D.; Schwarz, E.J.; Prockop, D.J.; Black, I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000, 61, 364–370. [Google Scholar] [CrossRef]
- Woodbury, D.; Reynolds, K.; Black, I.B. Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J. Neurosci. Res. 2002, 15, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Bertani, N.; Malatesta, P.; Volpi, G.; Sonego, P.; Perris, R. Neurogenic potential of human mesenchymal stem cells revisited: Analysis by immunostaining, time-lapse video and microarray. J. Cell Sci. 2005, 118, 3925–3936. [Google Scholar] [CrossRef] [PubMed]
- Neuhuber, B.; Gallo, G.; Howard, L.; Kostura, L.; Mackay, A.; Fischer, I. Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: Disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype. J. Neurosci. Res. 2004, 77, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Renuka, R.; Ma, D.F. Cytokine-induced stable neuronal differentiation of human bone marrow mesenchymal stem cells in a serum/feeder cell-free condition. Dev. Growth Differ. 2005, 47, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Hynes, M.; Porter, J.A.; Chiang, C.; Chang, D.; Tessier-Lavigne, M.; Beachy, P.A.; Rosenthal, A. Induction of midbrain dopaminergic neurons by sonic hedgehog. Neuron 1995, 15, 35–44. [Google Scholar] [CrossRef]
- Ye, W.; Shimamura, K.; Rubenstein, J.L.R.; Hynes, M.A.; Rosenthal, A. FGF and SHH signals control dopaminergic and serotonergic fate in the anterior neural plate. Cell 1998, 93, 755–766. [Google Scholar] [CrossRef]
- Roussa, E.; Farkas, L.M.; Krieglstein, K. TGF-β promotes survival on mesencephalic dopaminergic neurons in cooperation with Shh and FGF-8. Neurobiol. Dis. 2004, 16, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Pernaute, R.; Studer, L.; Ferrari, D.; Perrier, A.; Lee, H.; Vinuela, A.; Isacson, O. Long-term survival of dopamine neurons derived from parthenogenetic primate embryonic stem cells (Cyno-1) after transplantation. Stem Cells 2005, 23, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Minozzi, M.C.; Toffano, G.; Leon, A.; Skaper, S.D. Basic fibroblast growth factor promotes the survival and development of mesencephalic neurons in culture. Dev. Biol. 1989, 133, 140–147. [Google Scholar] [CrossRef]
- Beck, K.D.; Knusel, B.; Hefti, F. The nature of the trophic action of brain-derived neurotrophic factor, des(1-3)-insulin-like growth FACTOR-1, and basic fibroblast growth factor on mesencephalic dopaminergic neurons developing in culture. Neuroscience 1993, 52, 855–866. [Google Scholar] [CrossRef]
- Mayer, E.; Dunnett, S.B.; Pellitteri, R.; Fawcett, J.W. Basic fibroblast growth factor promotes the survival of embryonic ventral mesencephalic dopaminergic neurons-i. Effects in vitro. Neuroscience 1993, 56, 379–388. [Google Scholar] [CrossRef]
- Takayama, H.; Ray, J.; Raymon, H.K.; Baird, A.; Hogg, J.; Fisher, L.J.; Gage, F.H. Basic fibroblast growth factor increases dopaminergic graft survival and function in a rat model of Parkinson’s disease. Nat. Med. 1995, 1, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Timmer, M.; Cesnulevicius, K.; Winkler, C.; Kolb, J.; Lipokatic-Takacs, E.; Jungnickel, J.; Grothe, C. Fibroblast growth factor (FGF)-2 and FGF receptor 3 are required for the development of the substantia nigra, and FGF2 plays a crucial role for the rescue of dopaminergic neurons after 6-hydroxydopamine lesion. J. Neurosci. 2007, 27, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Hyman, C.; Hofer, M.; Barde, Y.A.; Juhasz, C.; Yancopoulos, G.D.; Squinto, S.P.; Lindsay, R.M. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 1991, 350, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Knusel, B.; Beck, K.D.; Winslow, J.W.; Rosenthal, A.; Burton, L.E.; Widmer, H.R.; Nikolics, K.; Hefti, F. Brain-derived neurotrophic factor administration protects basal forebrain cholinergic but not nigral dopaminergic neurons from degenerative changes after axotomy in the adult rat brain. J. Neurosci. 1992, 12, 4391–4402. [Google Scholar] [PubMed]
- Altar, C.A.; Boylan, C.B.; Fritsche, M.; Jones, B.E.; Jackson, C.; Wiegand, S.J.; Lindsay, R.M.; Hyman, C. Efficacy of brain-derived neurotrophic factor and neurotrophin-3 on neurochemical and behavioral deficits associated with partial nigrostriatal and dopamine lesions. J. Neurochem. 1994, 63, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Levivier, M.; Przedborski, S.; Bencsics, C.; Kang, U.J. Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson’s disease. J. Neurosci. 1995, 15, 7810–7820. [Google Scholar] [PubMed]
- Baquet, Z.C.; Bickford, P.C.; Jones, K.R. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J. Neurosci. 2005, 25, 6251–6259. [Google Scholar] [CrossRef] [PubMed]
- Tondreau, T.; Lagneaux, L.; Djeneffe, M.; Massy, M.; Mortier, C.; Delforge, A.; Bron, D. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation 2004, 72, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Lamoury, F.M.J.; Croitoru-Lamoury, J.; Brew, B.J. Undifferentiated mouse mesenchymal stem cells spontaneously express neural and stem cell markers Oct-4 and Rex-1. Cytotherapy 2006, 8, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Karaoz, E.; Aksoy, A.; Ayhan, S.; Sariboyaci, A.E.; Kaymaz, F.; Kasap, M. Characterization of mesenchymal stem cells from rat bone marrow: Ultrastructural properties, differentiation potential, and immunophenotypic markers. Histochem. Cell Biol. 2009, 132, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Foudah, D.; Redondo, J.; Caldara, C.; Carini, F.; Tredici, G.; Miloso, M. Expression of neural markers by undifferentiated rat mesenchymal stem cells. J. Biomed. Biothechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, J.; Fink, K.D.; Crane, T.A.; Davis, K.K.; Bombard, M.C.; Clerc, S.; Bavar, A.M.; Lowrance, S.A.; Song, C.; Witte, S.; et al. Reductions in behavioral deficits and neuropathology in the R6/2 mouse model of Huntington’s disease following transplantation of bone-marrow-derived mesenchymal stem cells is dependent on passage number. Stem Cell Res. Ther. 2015, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, C.; Lee, N.; Buchwalk, N.A.; Radisavljevic, Z.; Levine, M.S. Age-induced changes in electrophysiological responses of neostriatal neurons recorded in vitro. Neuroscience 1992, 51, 411–423. [Google Scholar] [CrossRef]
- Kuijlaars, J.; Oyelami, T.; Diels, A.; Rohrbacher, J.; Versweyveld, S.; Meneghello, G.; Tuefferd, M.; Verstraelen, P.; Detrez, J.R.; Verschuuren, M.; et al. Sustained synchronized neuronal network activity in a human astrocyte co-culture system. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Trzaska, K.A.; King, C.C.; Li, K.Y.; Kuzhikandathil, E.V.; Nowycky, M.C.; Ye, J.H.; Rameshwar, P. Brain-derived neurotrophic factor facilitates maturation of mesenchymal stem cell-derived dopamine progenitors to functional neurons. J. Neurochem. 2009, 110, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Kriks, S.; Shim, J.W.; Piao, J.; Ganat, Y.M.; Wakeman, D.R.; Xie, Z.; Carillo-Reid, L.; Auyeung, G.; Antonacci, G.; Buch, A.; et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011, 480, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Doi, D.; Samata, B.; Katsukawa, M.; Kikuchi, T.; Morizane, A.; Ono, Y.; Sekiguchi, K.; Nakagawa, M.; Parmar, M.; Takahashi, J. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Rep. 2014, 2, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Liao, M.C.; Chen, A.K.; Wei, S.; Xie, S.; Reuveny, S.; Zhou, Z.D.; Hunziker, W.; Tan, E.K.; Oh, S.K.W.; et al. Immature midbrain dopaminergic neurons derived from floor-plate method improve cell transplantation therapy efficacy for Parkinson’s disease. Stem Cells Transl. Med. 2017, 6, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Holzschuh, J.; Ryu, S.; Aberger, F.; Driever, W. Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech. Dev. 2001, 101, 237–243. [Google Scholar] [CrossRef]
- White, R.B.; Thomas, M.G. Moving beyond tyrosine hydroxylase to define dopaminergic neurons for use in cell replacement therapies. CNS Neurol. Disord. Drug Targets 2012, 11, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Pickel, V.M.; Joh, T.H.; Reis, D.J. Ultrastructural localization of tyrosine hydroxylase in noradrenergic neurons of brain. Proc. Natl. Acad. Sci. USA 1975, 72, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Augood, S.J.; Westmore, K.; McKenna, P.J.; Emson, P.C. Coexpression of dopamine transporter mRNA and tyrosine hydroxylase mRNA in ventral mesencephalic neurons. Mol. Brain Res. 1993, 20, 328–334. [Google Scholar] [CrossRef]
- Kim, K.S.; Seo, H.; Kim, C.; Hwang, D.Y. Transcriptional regulation of the dopamine β-hydroxylase gene. Adv. Behav. Biol. 2002, 53, 127–130. [Google Scholar]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Kitayama, S.; Walther, D.; Uhl, G. Dopamine transporter mRNA: Dense expression in ventral midbrain neurons. Mol. Brain Res. 2002, 13, 359–362. [Google Scholar] [CrossRef]
- Cerruti, C.; Walther, D.M.; Kuhar, M.J.; Uhl, G.R. Dopamine transporter mRNA expression is intense in rat midbrain neurons and modest outside midbrain. Mol. Brain Res. 1993, 18, 181–186. [Google Scholar] [CrossRef]
- Uhl, G.R.; Walthers, D.; Mash, D.; Fauceux, B.; Javoy-Agid, F. Dopamine transporter messenger RNA in Parkinson’s disease and control substantia nigra neurons. Ann. Neurol. 1994, 35, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Ciliax, B.J.; Heilman, C.; Demehyshyn, L.L.; Pristupa, Z.B.; Ince, E.; Hersch, S.M.; Niznik, H.B.; Lebey, A.I. The dopamine transporter: Immunochemical characterization and localization in the brain. J. Neurosci. 1995, 15, 1714–1723. [Google Scholar] [PubMed]
- Nirenberg, M.J.; Vaughan, R.A.; Uhl, G.R.; Kuhar, M.J.; Pickel, V.M. The dopamine transporter is localized to dendritic and axonal plasma membranes of the nigrostriatal dopaminergic neurons. J. Neurosci. 1996, 16, 436–447. [Google Scholar] [PubMed]
- Hersch, S.M.; Yi, H.; Heilman, C.J.; Edwards, R.H.; Levey, A.I. Subcellular localization and molecular topography of dopamine transporter in the striatum and substantia nigra. J. Comp. Neurol. 1997, 388, 211–227. [Google Scholar] [CrossRef]
- Counihan, T.; Penney, J. Regional dopamine transporter gene expression in the substantia nigra from control and Parkinson’s disease brains. J. Neurol. Neurosurg. Psychiatry 1998, 65, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Cohen, J.D. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef] [PubMed]
- German, D.C.; Manaye, K.F. Midbrain dopaminergic neurons (nuclei A8, A9, and A10): Three- dimensional reconstruction in the rat. J. Comp. Neurol. 1993, 331, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Grealish, S.; Jonsson, M.E.; Li, M.; Kirik, D.; Bjorklund, A.; Thompson, L.H. The A9 dopamine neuron component in grafts of ventral mesencephalon is an important determinant for recovery of motor function in a rat model of Parkinson’s disease. Brain 2010, 133, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, E.; Perlmann, T. Neuronal cell replacement in Parkinson’s disease. J. Intern. Med. 2009, 266, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Reyes, S.; Fu, Y.; Double, K.; Thompson, L.; Kirik, D.; Paxinos, G.; Halliday, G.M. GIRK2 expression in dopamine neurons of the substantia nigra and ventral tegmental area. J. Comp. Neurol. 2012, 520, 2591–2607. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Rolston, J.D.; Potter, S.M. How to culture, record and stimulate neuronal networks on micro-electrode arrays (MEAs). J. Vis. Exp. 2010. [Google Scholar] [CrossRef] [PubMed]
- Kozera, B.; Rapacz, M. Reference genes in real-time PCR. J. Appl. Genet. 2013, 54, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmitten, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Marker | P4 MSCs | P40 MSCs |
|---|---|---|
| CD90 | 965 | 36.2% |
| CD105 | 1.5% | 8.5% |
| MHC II | <0.1% | <0.1% |
| CD34 | <0.1% | <0.1% |
| TUJ1 | 49.5% | 31.55 |
| Nestin | 83.25% | 52.7% |
| Marker | P4 Control | P4 DDI | P4 IDI | P40 Control | P40 DDI | P40 IDI |
|---|---|---|---|---|---|---|
| DAT | 4.7% | 45.3% | 75.7% | 9.4% | 78.7% | 50.9% |
| TH | 16.9% | 32.1% | 19.1% | 10.2% | 35.8% | 17.8% |
| MAP2 | 0.9% | 15.8% | 66.0% | 1.4% | 13.1% | 9.1% |
| Nestin | 80.4% | 39.4% | 75.4% | 25.2% | 76.3% | 69.1% |
| lmx1a | 60.8% | 57.2% | 78.4% | 2.7% | 64.7% | 71.6% |
| Marker | P4 | P40 |
|---|---|---|
| DCX | 99.2% | 99.4% |
| Nestin | 91.6% | 84.8% |
| TUJ1 | 97.5% | 97.3% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shall, G.; Menosky, M.; Decker, S.; Nethala, P.; Welchko, R.; Leveque, X.; Lu, M.; Sandstrom, M.; Hochgeschwender, U.; Rossignol, J.; et al. Effects of Passage Number and Differentiation Protocol on the Generation of Dopaminergic Neurons from Rat Bone Marrow-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 720. https://doi.org/10.3390/ijms19030720
Shall G, Menosky M, Decker S, Nethala P, Welchko R, Leveque X, Lu M, Sandstrom M, Hochgeschwender U, Rossignol J, et al. Effects of Passage Number and Differentiation Protocol on the Generation of Dopaminergic Neurons from Rat Bone Marrow-Derived Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2018; 19(3):720. https://doi.org/10.3390/ijms19030720
Chicago/Turabian StyleShall, Gabrielle, Megan Menosky, Sarah Decker, Priya Nethala, Ryan Welchko, Xavier Leveque, Ming Lu, Michael Sandstrom, Ute Hochgeschwender, Julien Rossignol, and et al. 2018. "Effects of Passage Number and Differentiation Protocol on the Generation of Dopaminergic Neurons from Rat Bone Marrow-Derived Mesenchymal Stem Cells" International Journal of Molecular Sciences 19, no. 3: 720. https://doi.org/10.3390/ijms19030720






