1. Introduction
Avascular necrosis of the femoral head (ANFH) is a common and frequently-occurring disease, mainly occurring in adults aged 20–50 years old. The disability rate of ANFH is extremely high, seriously affecting the life quality and working capacity of patients, resulting in a huge burden for society and families. Around 30 million people worldwide are suffering from the disease, and the incidence is still growing.
Currently, there is no effective cure for ANFH [
1], and this has become the challenge to be jointly tackled by the world’s medical community. Interventional therapies, medullary core decompression, vascularized bone graft surgery, osteotomy, etc., have been used at the early stages (Ficat stage I and II), yet nearly all of them could not stop the development of disease. When advanced to stage III and IV, femoral head collapse could occur, and can be only rescued by artificial joint replacement, but with a failure rate of 10–50% within five years. Due to most people by this disease mainly being young adults, and by far the best artificial femoral head can only be used for 10–15 years, patients often must experience two or three replacements, which is a painful process with a heavy financial burden. Therefore, exploring new and effective early treatment and trying the best to preserve the patient’s own femoral head, is especially important for ANFH therapy. The bone marrow mesenchymal stem cell (BMSC) adoptive cell therapy has been extensively tested in clinical, and the improvement of the curative effect has been well recognized. BMSCs are injected through the hole resulting from the core decompression of the necrotic femoral head to take part in bone repair and regeneration [
1], on the one hand by secreting hepatocyte growth factor (HGF) and other nutritional factors, on the other hand by proliferating and differentiating into osteogenic cells in the local bone microenvironment composed by the bone marrow and the trabeculae of cancellous bone [
2]. Farzan M et al. compared the therapeutic efficacy of core decompression with or without BMSC transplantation in 28 hips with early-stage ANFH using the WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) questionnaire, VAS (visual analogue scale) pain index, and magnetic resonance imaging (MRI). Results showed that all patients achieved improved condition significantly (
p < 0.001) assessed by the mean WOMAC and VAS scores, while MRI examination indicated a significant improvement in hips with bone marrow stem cell injection (
p = 0.046) and significant worsening in hips without stem cell injection (
p < 0.001), confirming the effectiveness of using BMSCs in treatment of early ANFH [
3].
The exact mechanism of tissue repair mediated by BMSCs is not fully elucidated, but studies have shown that BMSC-secreted HGF and other neurotrophic factors have played important roles in this process [
4]. HGF is a pleiotropic cytokine and functions as a potent mitogen and histologic nutrition factor to promote repair in adult tissues, such as the liver [
5], heart [
6], and muscle [
7]. In addition, HGF also has pro-angiogenic effect that can improve the underlying cause of ANFH—the blood disorder—while inhibiting apoptosis and reducing fibrosis [
8,
9]. In vivo, HGF gene-modified BMSCs significantly accelerated the myocardial tissue repair of myocardial infarction after trauma compared with un-transfected BMSCs [
10], while BMSCs with HGF gene knocked out could not improve blood vessel regeneration in the limb ischemia model [
11]. We verified the therapeutic efficacy of HGF gene-modified BMSC treatment on ANFH in the rabbit model, and further explored the mechanisms. Our data indicated the difference in the activation of downstream different signaling pathways of the HGF receptor c-MET by different doses of HGF, resulting in distinct biological effects: in osteogenic environment, 100 ng/mL of HGF induced higher level of ERK1/2 pathway activation, which can significantly promote BMSC proliferation and inhibit osteogenic differentiation; while 20 ng/mL of HGF activated the AKT pathway with higher level than its effects on the ERK1/2 pathway activation, thus prompting BMSCs to differentiate into osteoblasts. This change in exogenous expression of HGF made genetically-modified BMSCs show different characteristics of life activities at different stages of treatment, at early stage cells bloomed so as to achieve the number required for organization repair, then differentiated into osteogenic cells in the bone microenvironment, playing an effective role in tissue repair [
12]. However, whether the above founding in rabbit BMSCs also works with the similar effects in human BMSCs is unknown.
C-MET is a receptor tyrosine kinase (RTKs) and essential for embryonic development and wound healing, with HGF as the only known ligand. Activation of downstream signal pathways mediated different biological effects of c-MET. Among them, the RAS pathway mediates HGF-induced cell dispersion and proliferation signals, resulting in branching morphogenesis [
13]. Different from most mitogens, HGF induces sustained RAS activation, and thus prolonged activation of MAPK, together with the STAT pathway played a key role in mediating cell invasive growth and morphogenetic processes [
14]. Activation of PI3K pathway is through two approaches: it is downstream molecules of RAS, and also directly recruited by the cohesion protein binding sites on the c-MET [
15]. PI3K activation also triggers a survival signal activated by the AKT pathway activation [
16]. C-MET downstream signal pathways also include the β-catenin pathway. The β-catenin pathway is a classic key member of the WNT signaling pathway, translocating into the nucleus following c-MET activation and involving in the transcriptional regulation of a large number of genes [
17]. Classic WNT signaling pathway promotes osteoblastic differentiation of BMSCs early in osteogenesis by relying on or not relying on the osteogenic differentiation of key transcription factor runt-related transcription factor 2 (Runx2) [
18]. Clearly, each of signal pathways coordinated the control on cell activity. Therefore, fully aware of the effects of different HGF levels on the c-MET downstream signaling pathways related to osteogenic differentiation and proliferation will provide theoretical support for a more rational use of HGF to promote BMSC effect on bone tissue repair in osteogenic environments.
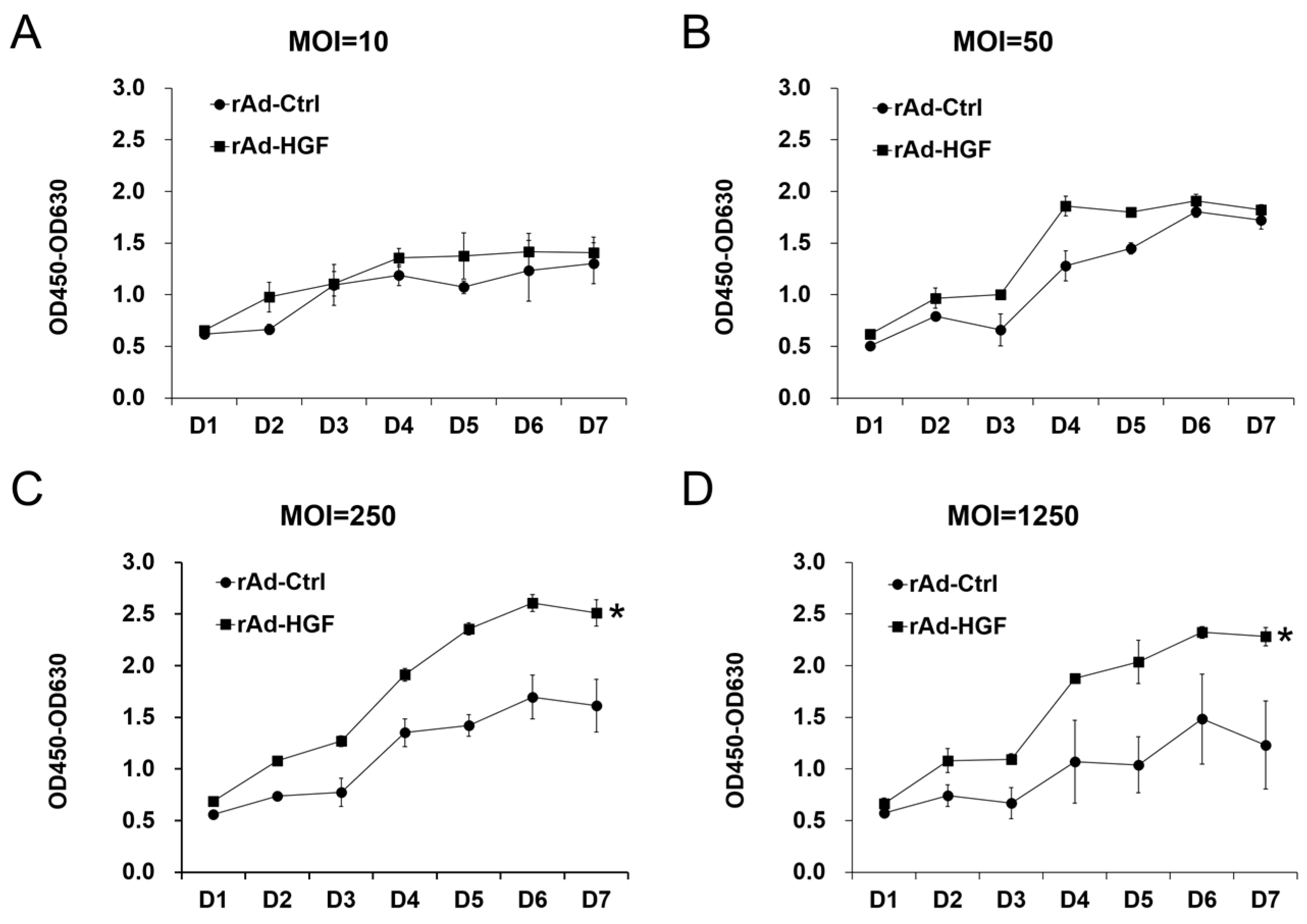
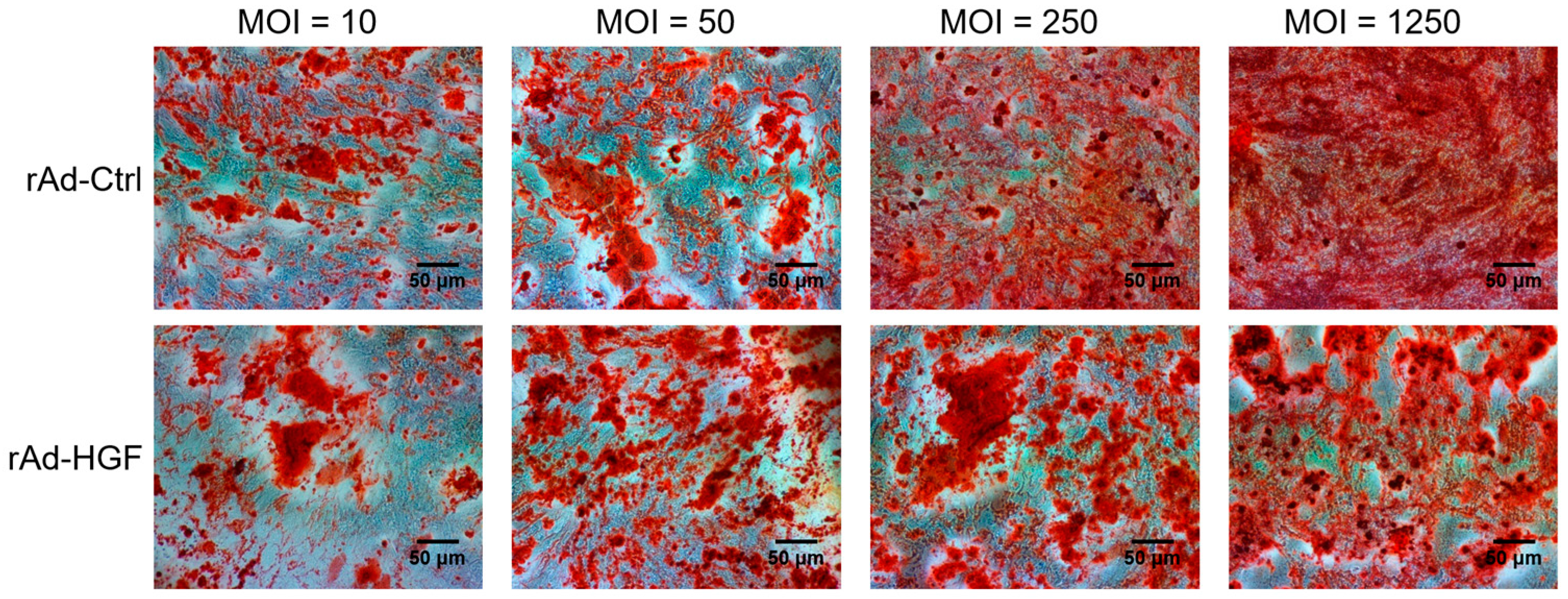
In this study, we generated HGF-gene modified hBMSCs using rAd-HGF and tested the effects of low MOI (MOI = 10 or 50) and high MOIs (MOI = 250 or 1250) on the expression of HGF which was thought to be critical regulator in ANFH therapy. Our results indicated that higher MOIs of rAd-HGF (MOI = 250 or 1250) not only significantly promote HGF expression, but also play stronger roles in enhancing hBMSC proliferation and osteogenic differentiation than rAd-Ctrl and low MOIs of rAd-HGF (MOI = 10 or 50). Inhibitor-specific blockage of signaling pathways showed that the WNT signaling pathway plays anti-osteogenic roles while the PI3K/AKT pathway is pro-osteogenic for hBMSCs. Our findings not only indicated the optimized MOI of rAd-HGF for preparing HGF-gene modified hBMSCs, but also suggested that the WNT pathway is a promising inhibitory target for treatment of ANFH using rAd-HGF-modified hBMSCs.
3. Discussion
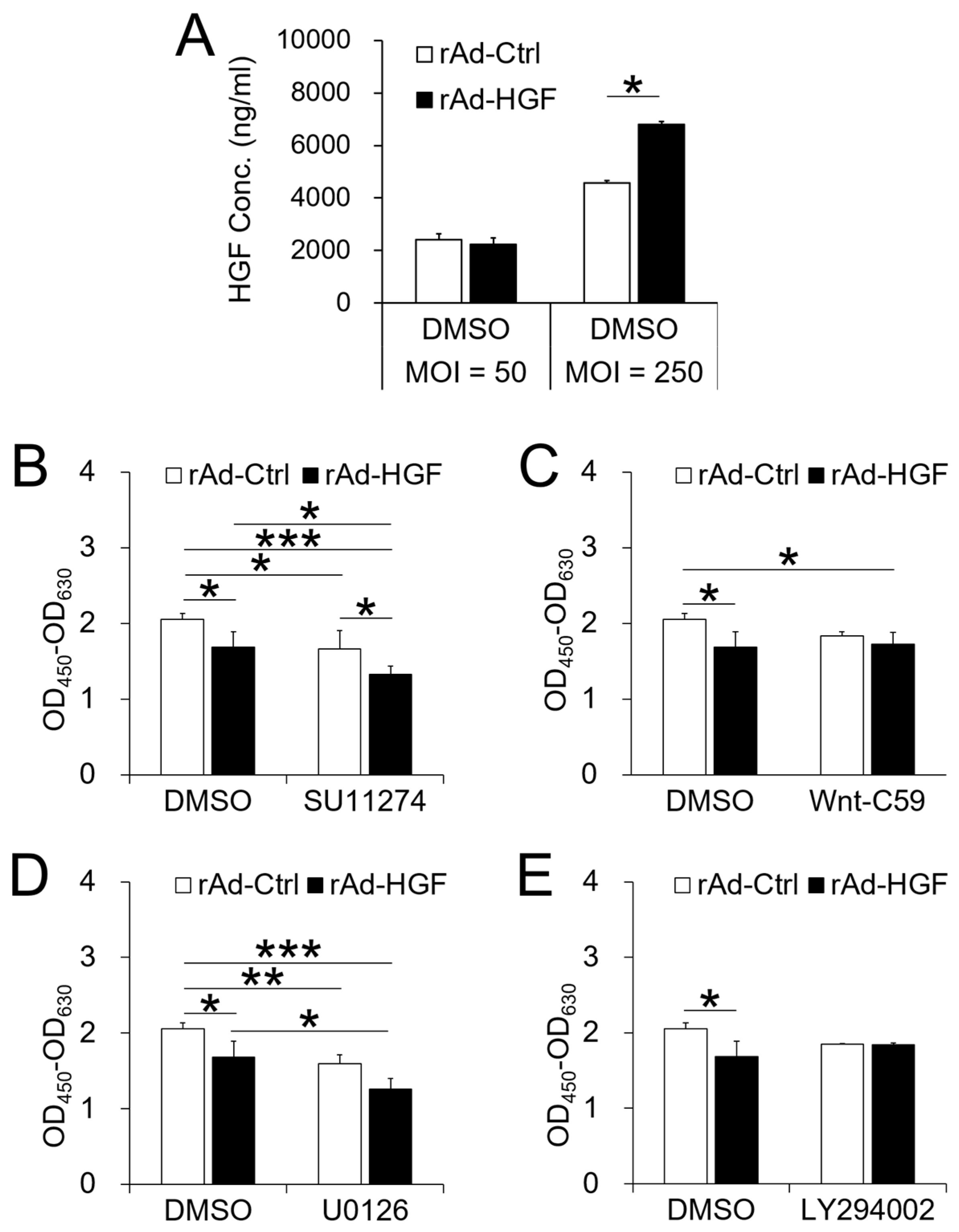
BMSC adoptive cell therapy is a promising approach for ANFH. As a self-secreted cytokine, pleiotropic HGF has been demonstrated to play important roles in BMSC activity. In this study, the MOI-dependent effects of rAd-HGF infection for HGF expression on hBMSC proliferation and osteogenic differentiation have been explored, while the roles of c-MET-related signaling pathways are also investigated. Our results indicated that higher MOIs of rAd-HGF (MOI = 250 or MOI = 1250) not only significantly promoted HGF expression, but also played stronger roles to enhance hBMSC proliferation and osteogenic differentiation than rAd-Ctrl and low MOIs of rAd-HGF (MOI = 10 or MOI = 50) did. As MOI 250 showed the strongest effects in producing HGF and promoting both proliferation and osteogenesis of hBMSCs, it was selected for further evaluating the influences of HGF receptor c-MET-related signaling pathways in hBMSC proliferation and osteogenesis. Interestingly, inhibitor-treatment experiments showed that the WNT pathway plays anti-osteogenic roles while the PI3K/AKT pathway is pro-osteogenic for hBMSCs.
Signaling pathways play complex roles in cell bioactivity, also in that of hBMSCs in osteogenic differentiation environment as we demonstrated in this study. As the receptor of HGF on BMSCs, c-MET suppressed by SU11274 treatment significantly inhibited the proliferation of cells infected with both recombinant adenoviruses compared with DMSO-treated cells, and that of cells infected with rAd-HGF compared with cells infected with rAd-Ctrl, indicating that HGF promotes hBMSC proliferation through activating c-MET signaling. In addition, as expected, the ERK1/2 pathway activated by HGF functioned as a remarkable enhancing factor to promote hBMSC proliferation in the osteogenic differentiation condition. On the contrary, WNT signaling has been reported to regulate transcription of multiple genes, including the RUNX2 which promotes BMSC osteogenic differentiation [
18]. For this reason, in this study, the WNT pathway was supposed to enhance BMSC osteogenic differentiation and possibly inhibit BMSC proliferation. However, when treated with Wnt-C59 to inhibit the WNT signaling, no effects on cell proliferation were observed when compared the DMSO-treated cells and the inhibitor-treated cells infected with the same recombinant adenovirus. The only difference appeared between Wnt-C59-treated and rAd-HGF-infected cells and DMSO-treated and rAd-Ctrl-infected cells (
p < 0.05). These results indicated that the WNT pathway exerted no effects in hBMSC proliferation in the osteogenic medium. Similarly, the PI3K/AKT pathway also showed few effects on cell proliferation. These observations revealed that the c-MET and the ERK1/2 signaling pathways are involved in promotion of hBMSC proliferation in the osteogenic differentiation medium. However, rAd-HGF infection could inhibit these two pathways to suppress hBMSC proliferation under this condition.
It is well known that cell proliferation and differentiation are two opposite biological activities and regulated by different but maybe interactive signaling pathways. In this study, our results indicated that c-MET mainly promoted proliferation of hBMSCs. However, at the same time, inhibiting c-MET eliminated the promotive effects of rAd-HGF infection on hBMSC osteogenesis, indicating that c-MET also contributed to hBMSC differentiation. However, when cells were infected with rAd-Ctrl, treatment with SU11274 could greatly promote cell osteogenesis. Because BMSCs could self-secret HGF, this result suggested that the regulatory effects of c-MET were obvious at low dose of HGF, thus inhibiting c-MET not only inhibited cell proliferation, but also enhanced hBMSC osteogenic differentiation induced by self-secreted HGF. Meanwhile, consistent with the activity to promote cell proliferation, the ERK1/2 pathway exerted few effects on osteogenesis. These results indicated that the two cellular activities, proliferation and differentiation, indeed are opposite to each other. Interestingly, inhibition of the WNT pathway significantly promoted cell osteogenesis, contrary to the previous report on its promotive role in osteogenesis [
18]. When rAd-HGF-infected hBMSCs were treated with Wnt-C59, the osteogenic degree was significantly higher than rAd-Ctrl-infected cells treated with DMSO (
p < 0.001). Meanwhile, rAd-HGF-infected cells treated with DMSO showed comparable osteogenic degree when compared with rAd-Ctrl-infected cells treated with Wnt-C59, which suggested the HGF may be an inhibitory factor of the WNT pathway to promote hBMSC osteogenesis. In the meantime, as expected, our results indicated that the PI3K/AKT pathway indeed promoted hBMSC osteogenic differentiation. Treatment with LY294002 could greatly suppress the level of hBMSC osteogenesis elevated by rAd-HGF infection. Furthermore, similar osteogenic degrees between rAd-Ctrl-infected cells treated with DMSO and rAd-HGF-infected cells treated with LY294002 indicated that LY294002 could counteract the pro-osteogenic effects of the PI3K/AKT pathway after HGF activation. In summary, in rAd-HGF-infected hBMSCs, HGF induced activation of the PI3K/AKT pathway and inhibition of the WNT pathway functioned together to promote cell osteogenesis.
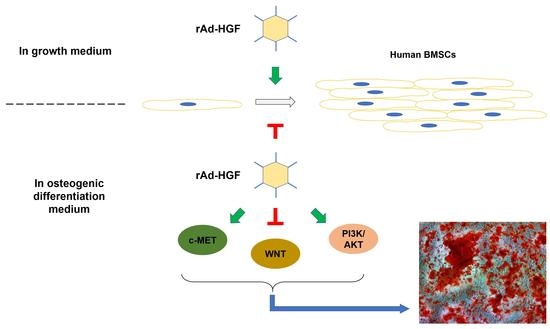
Here, we demonstrated the MOI-dependent effects of rAd-HGF on HGF expression and the proliferation and osteogenesis of hBMSCs, as well as the roles of various signaling pathways in rAd-HGF-mediated hBMSC proliferation and osteogenesis. Besides the positive regulatory roles of rAd-HGF with MOI = 250 in osteogenesis of hBMSCs, results of pathway-specific inhibitor treatments also demonstrated that the WNT pathway plays anti-osteogenic roles while the PI3K/AKT pathway is pro-osteogenic for hBMSCs. Our finding suggest that combinatory application of high MOI rAd-HGF (MOI = 250) with inhibitor of WNT pathway, like Wnt-C59, is a promising modality for the better application of HGF gene-modified hBMSCs in ANFH therapy.
4. Materials and Methods
4.1. Cell Cultures
Human bone marrow mesenchymal stem cells (hBMSCs) were bought from Cyagen Biosciences Inc., (Goleta, CA, USA). Informed consent was obtained in accordance with the Declaration of Helsinki and the Institutional Review Board of the Southern Medical University (approval number SMU-2015123, 2016.01.10). Cells were cultured at 37 °C, 5% CO2, in the Human Mesenchymal Stem Cell Basal Medium complemented with 10% of the qualified fetal bovine serum (FBS), 1% Penicillin-streptomycin and 1% Glutamine (all from Cyagen Biosciences Inc.). The potency of osteogenic, chondrogenic, and adipogenic differentiation has been confirmed before obtainment. Cells were infected with recombinant adenoviruses and the proliferation and osteogenic differentiation were assayed. In some experiments, cells were treated with signaling pathway inhibitors agents as indicated in the figure legends, including c-MET inhibitor SU11274 (10 μM), WNT inhibitor Wnt-C59 (2 μM), ERK1/2 inhibitor U0126 (10 μM), PI3K/AKT inhibitor LY294002 (10 μM) (all from Selleckchem, Houston, TX, USA), and DMSO (Sigma-Aldrich, St. Louis, MO, USA) was used as the negative control.
4.2. Preparation of Recombinant Adenoviruses
Recombinant adenovirus carrying HGF gene (rAd-HGF) and empty adenovirus (rAd-Ctrl) were constructed, packaged, purified, and the titer was detected as described previously [
21]. Prepared adenoviruses were aliquoted and stored at −80 °C before use.
4.3. ELISA
The supernatants of hBMSCs following treatments were collected and centrifuged at 3000× g, 4 °C for 5 min. The obtained supernatants were stored at −80 °C before assays. Secretion of HGF by hBMSCs were detected using ELISA kits (ExCell Bio, Shanghai, China) according to the protocols provided by the manufacturers. Considering the self-secretion of HGF by BMSCs, the results were deducted from the background.
4.4. Cell Viability
Human BMSCs were inoculated at 2.5–4 × 104/cm2 in 96-well plates (ExCell Bio) and cultured overnight. Cells were treated as indicated in the figure legends and cell viability was assayed at indicated time using WST-8 method (Cell Counting Kit-8; Dojindo, Kumamoto, Japan) according to the manufacturer’s instructions.
4.5. Osteogenic Differentiation and Calcium Accumulation Assay
Human BMSCs were inoculated at 2.5–4 × 10
4/cm
2 in 24-well plates. When the culture reached 100% confluence, the medium was changed to the Human Mesenchymal Stem Cell Osteogenic Differentiation Basal Medium complemented with 10% FBS, 1% Penicillin-streptomycin, 1% Glutamine, 0.2 mM ascorbate, 10 mM β-glycerophosphate, and 0.1 μM dexamethasone (all from Cyagen Biosciences Inc.). Media were changed every two days and the cells were cultured for 14–21 days. The calcium accumulation was assessed using alizarin red sulfate (AR-S; Cyagen Biosciences Inc.) staining and quantified as described previously [
12].
4.6. Western Blot
At different time post treatments as indicated in the legends, hBMSCs were lysed using RIPA lysis buffer containing 10% protease inhibitor complex (Roche Applied Science, Mannheim, Germany), 10% PhosSTOP Phosphatase Inhibitor Cocktail (Roche) and 1 μM DL-Dithiothreitol (Sigma-Aldrich). Western blot was performed using following antibodies: phosphorylated-c-Met (D26; 1:2000), c-Met (25H2; 1:2000), phosphorylated-Akt (p-Akt) (D9E; 1:2000), Akt (C67E7; 1:2000), ERK2 (C-14; 1:2000), phosphorylated-ERK1/2 (p-ERK1/2) (E-4; 1:1000) (Cell Signaling Technology, Inc., Beverly, MA, USA), non-phospho (active)-β-catenin (D2U8Y; 1:1000), GAPDH (1:2000; Zhongshan Goldenbridge Biotechnology Co., Ltd., Beijing, China), and appropriate horseradish peroxidase–conjugated secondary antibodies (1:5000; Zhongshan Goldenbridge). The SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used to develop the membranes according to manufacturer’s instructions. Results were observed and obtained using FluorChem Q Multi-Functional Imaging and Analysis System (ProteinSimple Ltd., San Jose, CA, USA).
4.7. Statistical Analysis
Data are expressed as the mean ± SD. The statistical significance was determined using one-way ANOVA. Post hoc multiple comparisons were performed using least significant difference or Dunnett’s T3. Differences with p < 0.05 were statistically significant. All statistical analyses were performed with SPSS statistical software version 16.0 (SPSS, Chicago, IL, USA).