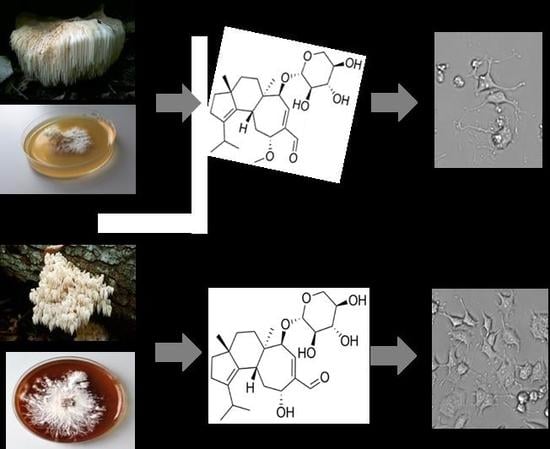
Two New Cyathane Diterpenoids from Mycelial Cultures of the Medicinal Mushroom Hericium erinaceus and the Rare Species, Hericium flagellum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Secondary Metabolites of Hericium erinaceus and Hericium flagellum
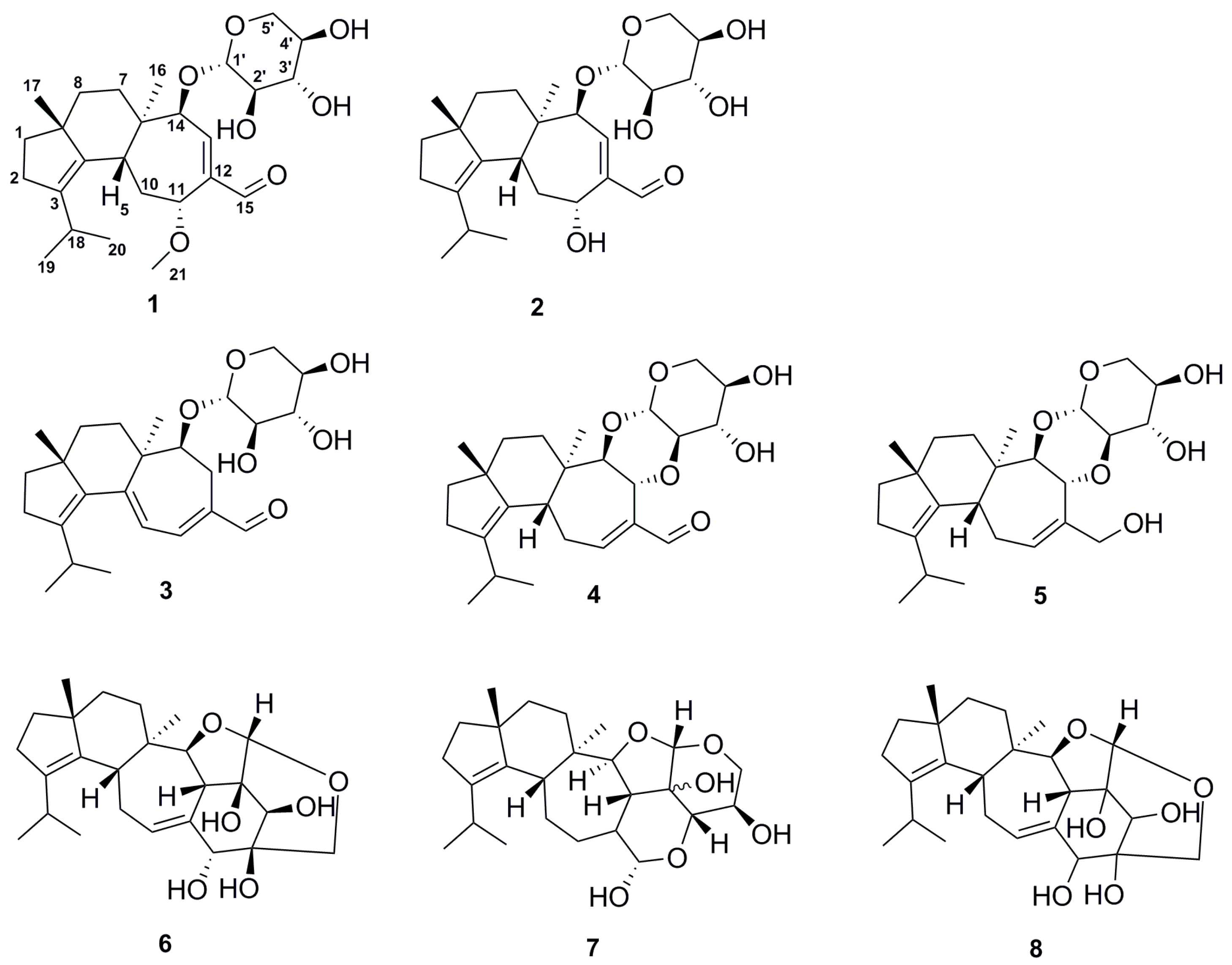
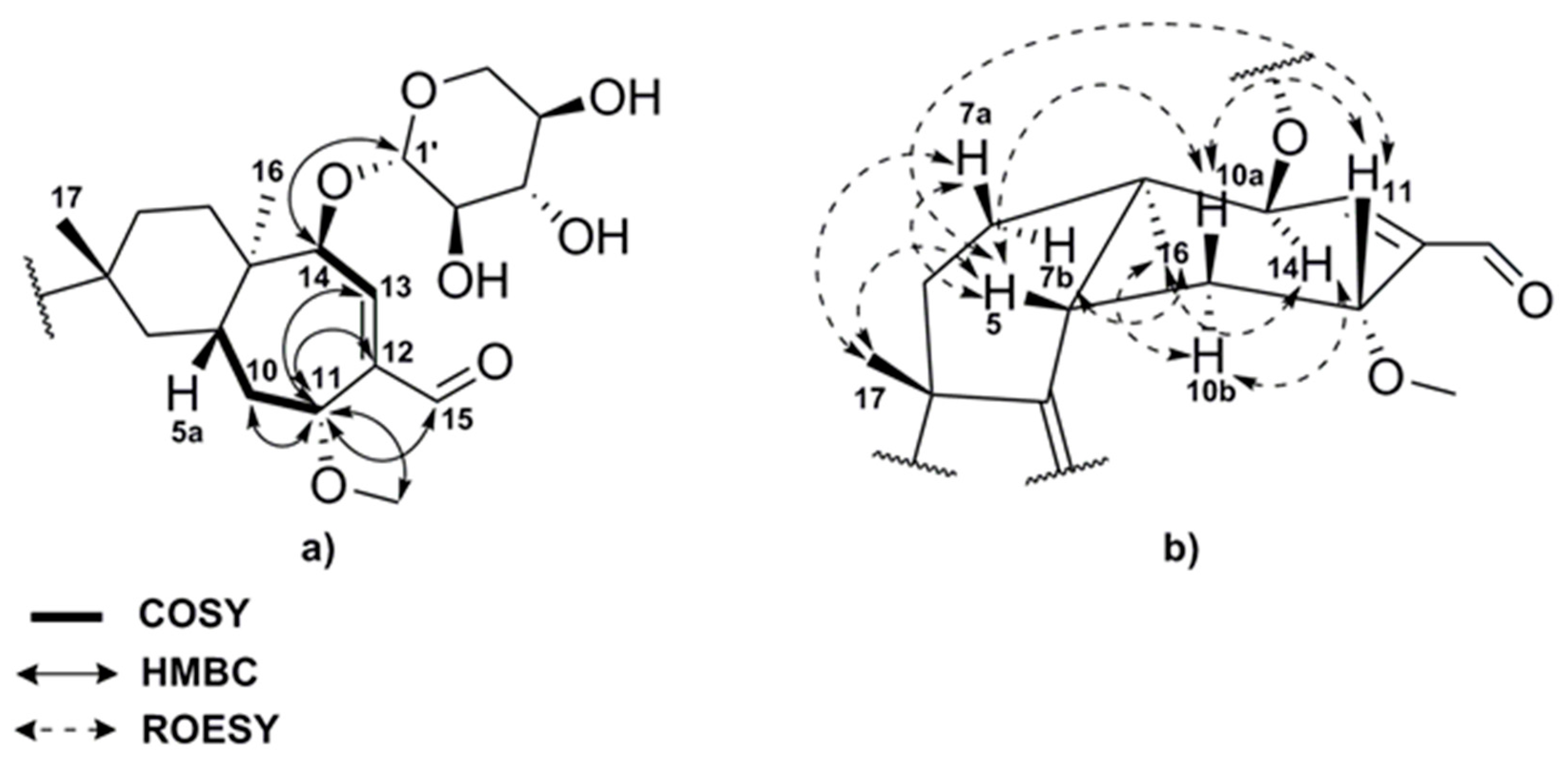
2.2. Structure Elucidation of Compounds 1–8
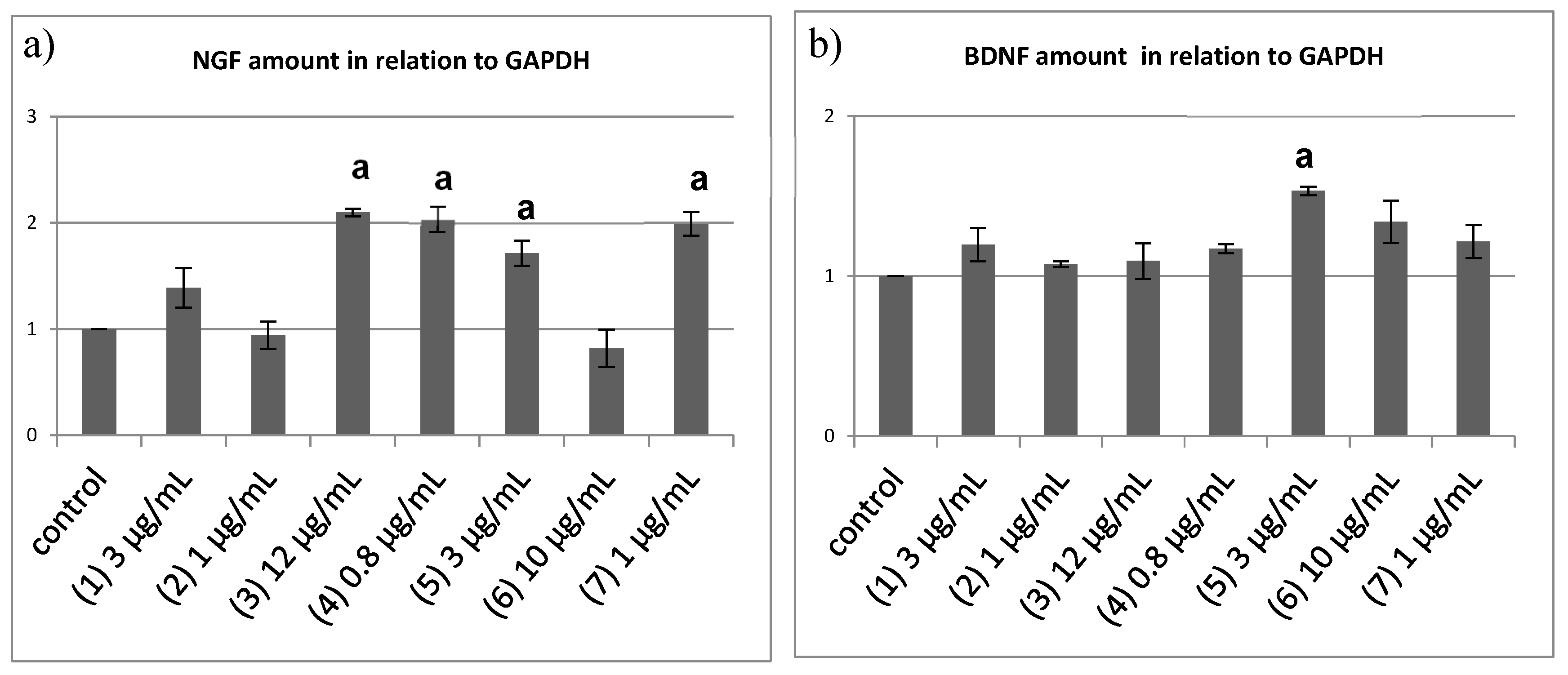
2.3. NGF-Induced Neurite Outgrowth in PC12 Cells
2.4. Semi-Quantitative Reverse Transcriptase PCR to Quantify the Neurotrophin mRNA Level
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material and Fermentation
3.3. Extraction and Isolation
3.4. Differentiation Assay
3.5. Reverse Transcriptase PCR
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NGF | Nerve growth factor |
| BDNF | Brain derived neurothrophic factor |
| ESI-MS | Electrospray ionization-mass spectrometry |
| NMR | Nuclear Magnetic Resonance |
| HR-ESI-MS | High resolution-Electrospray ionization-mass spectrometry |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| ZM | Sugar medium |
| SP | Soy peptone |
| IR | Infrared |
| DAD | Diode array detector |
| LC | Liquid chromatography |
| EDTA | Ethylene-diamine-tetra-acetic acid |
| DMEM | Dulbecco’s modified eagle’s medium |
| RPMI | Roswell park memorial institute medium |
References
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Harzdorf, N.; Khaing, Z.; Kang, D.; Camelio, A.M.; Shaw, T.; Schmidt, C.E.; Siegel, D. Neuronal growth promoting sesquiterpene–neolignans; syntheses and biological studies. Org. Biomol. Chem. 2012, 10, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, A.M.; Bianco, M.R.; Vitagliano, L.; Cavaliere, C.; Cirillo, G.; De Gioia, L.; Diana, D.; Colombo, D.; Redaelli, C.; Zaccaro, L.; et al. A new nerve growth factor-mimetic peptide active on neuropathic pain in rats. J. Neurosci. 2008, 28, 2698–2709. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ishibashi, M.; Satake, M.; Chen, X.; Oshima, Y.; Ohizumi, Y. Sterol and triterpenoid constituents of Verbena littoralis with NGF-potentiating activity. J. Nat. Prod. 2003, 66, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Wittstein, K.; Rascher, M.; Rupcic, Z.; Löwen, E.; Winter, B.; Köster, R.W.; Stadler, M. Corallocins A–C, nerve growth and brain-derived neurotrophic factor inducing metabolites from the mushroom Hericium coralloides. J. Nat. Prod. 2016, 79, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Shimada, A.; Shirai, R.; Okamoto, K.; Ojima, F.; Sakamoto, H.; Ishiguro, Y.; Furukawa, S. Erinacines A, B and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1994, 35, 1569–1572. [Google Scholar] [CrossRef]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91–113. [Google Scholar] [CrossRef]
- Chen, C.C.; Tzeng, T.T.; Chen, C.C.; Ni, C.L.; Lee, L.Y.; Chen, W.P.; Shiao, Y.J.; Shen, C.C. Erinacine S, a rare sesterterpene from the mycelia of Hericium erinaceus. J. Nat. Prod. 2016, 79, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Masui, A.; Tokuyama, S.; Nakamura, T. Erinacines J and K from the mycelia of Hericium erinaceum. Tetrahedron 2006, 62, 8463–8466. [Google Scholar] [CrossRef]
- Tang, H.Y.; Yin, X.; Zhang, C.C.; Jia, Q.; Gao, J.M. Structure diversity, synthesis, and biological activity of cyathane diterpenoids in higher fungi. Curr. Med. Chem. 2015, 22, 2375–2391. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Shimada, A.; Hosokawa, S.; Mori, H.; Sakamoto, H.; Ishiguro, Y.; Sakemi, S.; Bordner, J.; Kojima, N.; Furukawa, S. Erinacines E, F, and G, stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1996, 37, 7399–7402. [Google Scholar] [CrossRef]
- Kenmoku, H.; Sassa, T.; Kato, N. Isolation of erinacine P, a new parental metabolite of cyathane-xylosides, from Hericium erinaceum and its biomimetic conversion into erinacines A and B. Tetrahedron Lett. 2000, 41, 4389–4393. [Google Scholar] [CrossRef]
- Zhang, C.C.; Cao, C.Y.; Kubo, M.; Harada, K.; Yan, X.T.; Fukuyama, Y.; Gao, J.M. Chemical constituents from Hericium erinaceus promote neuronal survival and potentiate neurite outgrowth via the TrkA/Erk1/2 pathway. Int. J. Mol. Sci. 2017, 18, 1659. [Google Scholar] [CrossRef] [PubMed]
- Alcántara, S.; Frisen, J.; del Rio, J.A.; Soriano, E.; Barbacid, M.; Silos-Santiago, I. TrkB signaling is required for postnatal survival of CNS neurons and protects hippocampal and motor neurons from axotomy-induced cell death. J. Neurosci. 1997, 17, 3623–3633. [Google Scholar] [PubMed]
- Ahmed, S.; Reynolds, B.A.; Weiss, S. BDNF enhances the differentiation but not the survival of CNS stem cell-derived neuronal precursors. J. Neurosci. 1995, 15, 5765–5778. [Google Scholar] [PubMed]
- Noumeur, S.R.; Helaly, S.E.; Jansen, R.; Gereke, M.; Stradal, T.E.B.; Harzallah, D.; Stadler, M. Preussilides A–F, bicyclic polyketides from the endophytic fungus Preussia similis with antiproliferative activity. J. Nat. Prod. 2017, 80, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Chepkirui, C.; Matasyoh, J.C.; Decock, C.; Stadler, M. Two cytotoxic triterpenes from cultures of a Kenyan Laetiporus sp. (Basidiomycota). Phytochem. Lett. 2017, 20, 106–110. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Chyau, C.C.; Chen, C.C.; Lee, L.Y.; Chen, W.P.; Liu, J.L.; Lin, W.H.; Mon, M.C. Erinacine A-enriched Hericium erinaceus mycelium produces antidepressant-like effects through modulating BDNF/PI3K/Akt/GSK-3β signaling in mice. Int. J. Mol. Sci. 2018, 19, 341. [Google Scholar] [CrossRef] [PubMed]

| Compounds | H. erinaceus | H. flagellum |
|---|---|---|
| Erinacine Z1 (1) | + | − |
| Erinacine Z2 (2) | − | + |
| Erinacine A (3) | + | + |
| Erinacine B (4) | + | + |
| Erinacine C (5) | + | + |
| Erinacine E (6) | + | + |
| CJ14.258 (7) | − | + |
| Erinacine F (8) | − | + |
| Position | (1) | (2) | ||
|---|---|---|---|---|
| δC, Type | δH, m (J in Hz) | δC, Type | δH, m (J in Hz) | |
| 1 | 38.5, CH2 | 1.46, m; 1.62, m | 38.3, CH2 | 1.54, m |
| 2 | 28.4, CH2 | 2.27, m | 29.1, CH2 | 2.31, m |
| 3 | 139.9, C | 140.7, C | ||
| 4 | 136.6, C | 139.8, C | ||
| 5 | 40.4, CH | 1.93, m | 36.1, CH | 3.47, m |
| 6 | 40.6, C | 44.5, C | ||
| 7a | 30.4, CH2 | 1.32, td (12.2, 6.6) | 34.3, CH2 | 1.16, m |
| 7b | 1.62, m | 2.41, td (13.8, 4.2) | ||
| 8 | 37.0, CH2 | 1.46, m | 37.1, CH2 | 1.44, ddd (12.5, 7.7, 3.0) 1.54, m |
| 9 | 49.2, C | 50.4, C | ||
| 10b | 30.1, CH2 | 1.81, ddd (14.2, 11.9, 5.5) | 35.0, CH2 | 2.07, m |
| 10a | 2.54, dd (13.7, 10.1) | 2.14, m | ||
| 11 | 72.4, CH | 4.63, m | 62.1, CH | 4.78, br d (5.2) |
| 12 | 138.6, C | 148.2, C | ||
| 13 | 158.2, CH | 6.94, dd (5.6, 1.2) | 154.6, CH | 7.01, dd (7.3, 0.9) |
| 14 | 85.4, CH | 4.61, d (5.6) | 85.3, CH | 3.96, d (7.3) |
| 15 | 192.9, CH | 9.54, s | 194.2, CH | 9.43, s |
| 16 | 16.4, CH3 | 0.99, s | 16.4, CH3 | 0.82, s |
| 17 | 24.5, CH3 | 0.93, s | 24.7, CH3 | 1.14, s |
| 18 | 27.0, CH | 2.85, m | 27.7, CH | 3.08, m |
| 19 | 21.5, CH3 | 0.97, d (6.8) | 22.0, CH3 | 0.97, m |
| 20 | 21.8, CH3 | 0.97, d (6.8) | 22.3, CH3 | 0.97, m |
| 21 | 56.6, CH3 | 3.26, s | - | |
| 1′ | 105.3, CH | 4.34, d (7.0) | 106.3, CH | 4.56, d (5.6) |
| 2′ | 73.5, CH | 3.47, m | 74.6, CH | 3.51, m |
| 3′ | 69,6, CH | 3.74, td (8.8, 5.1) | 70.3, CH | 3.51, m |
| 4′ | 75.7, CH | 3.54, dd (8.8) | 73.3, CH | 3.41, dd (7.3, 5.8) |
| 5′ | 65.1, CH2 | 3.29, m 3.99, dd (11.7, 5) | 65.0, CH2 | 3.28, dd (11.8, 7.5) 3.88, dd (11.8, 3.3) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupcic, Z.; Rascher, M.; Kanaki, S.; Köster, R.W.; Stadler, M.; Wittstein, K. Two New Cyathane Diterpenoids from Mycelial Cultures of the Medicinal Mushroom Hericium erinaceus and the Rare Species, Hericium flagellum. Int. J. Mol. Sci. 2018, 19, 740. https://doi.org/10.3390/ijms19030740
Rupcic Z, Rascher M, Kanaki S, Köster RW, Stadler M, Wittstein K. Two New Cyathane Diterpenoids from Mycelial Cultures of the Medicinal Mushroom Hericium erinaceus and the Rare Species, Hericium flagellum. International Journal of Molecular Sciences. 2018; 19(3):740. https://doi.org/10.3390/ijms19030740
Chicago/Turabian StyleRupcic, Zeljka, Monique Rascher, Sae Kanaki, Reinhard W. Köster, Marc Stadler, and Kathrin Wittstein. 2018. "Two New Cyathane Diterpenoids from Mycelial Cultures of the Medicinal Mushroom Hericium erinaceus and the Rare Species, Hericium flagellum" International Journal of Molecular Sciences 19, no. 3: 740. https://doi.org/10.3390/ijms19030740