Polymeric Nano-Micelles as Novel Cargo-Carriers for LY2157299 Liver Cancer Cells Delivery
Abstract
:1. Introduction
2. Results
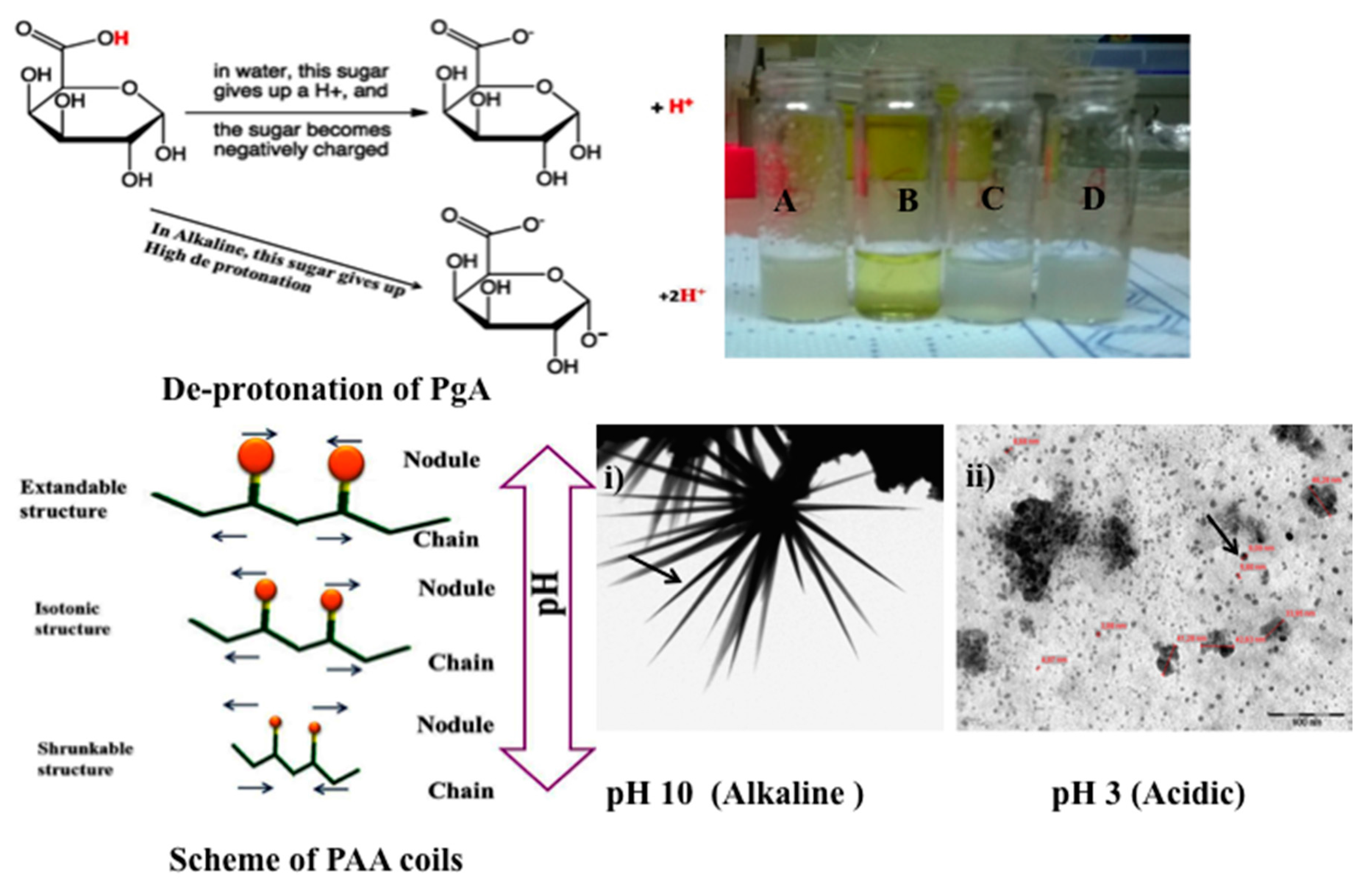
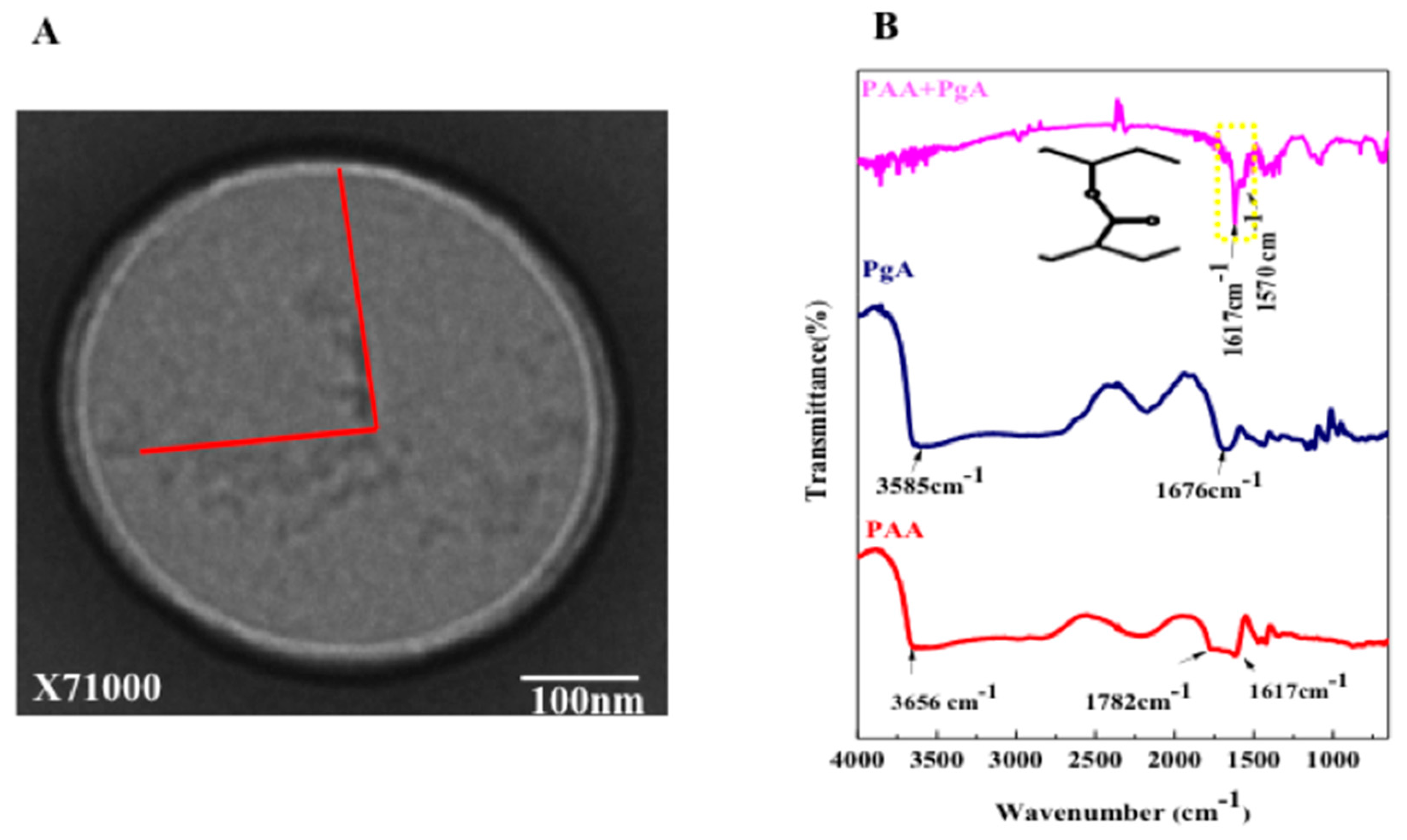
2.1. Physical Properties of PgA and PAA
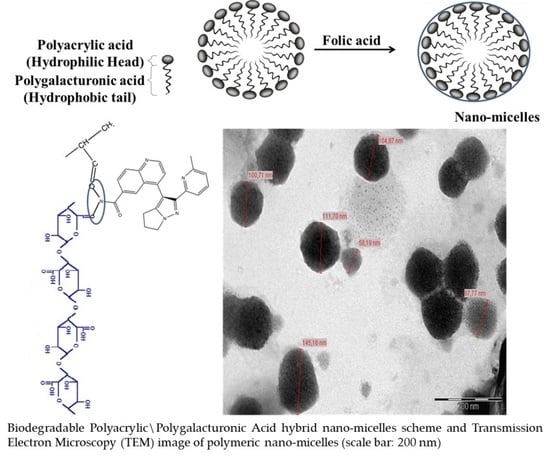
2.2. Synthesis of Nano-Micelles
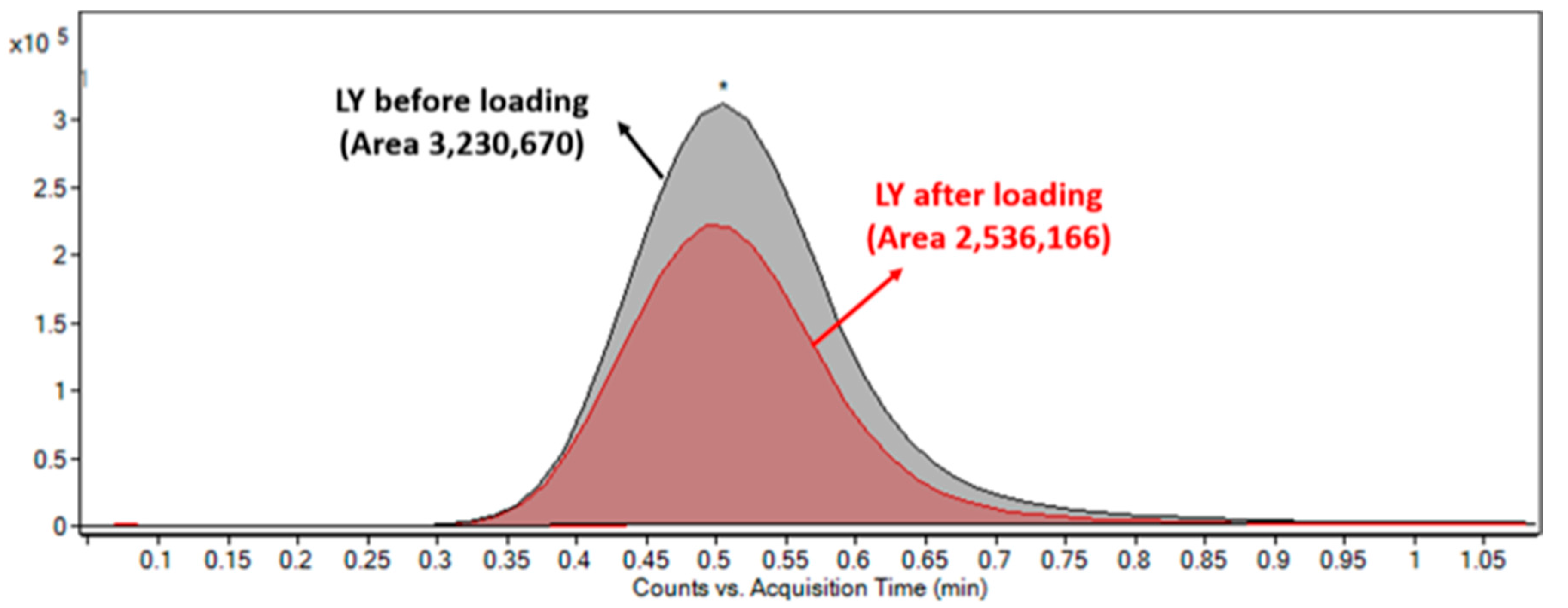
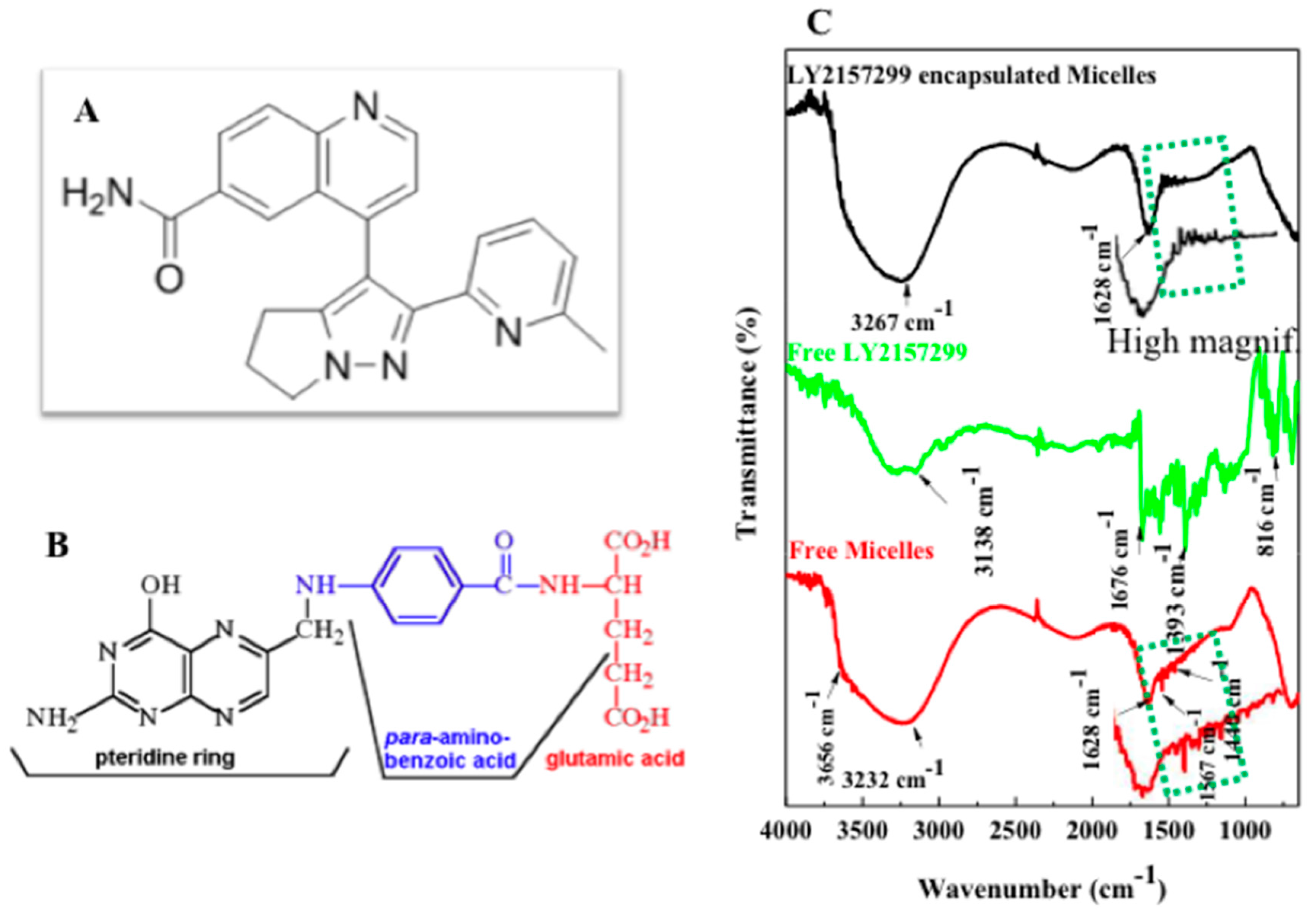
2.3. LY2157299 Loaded Nano-Micelles
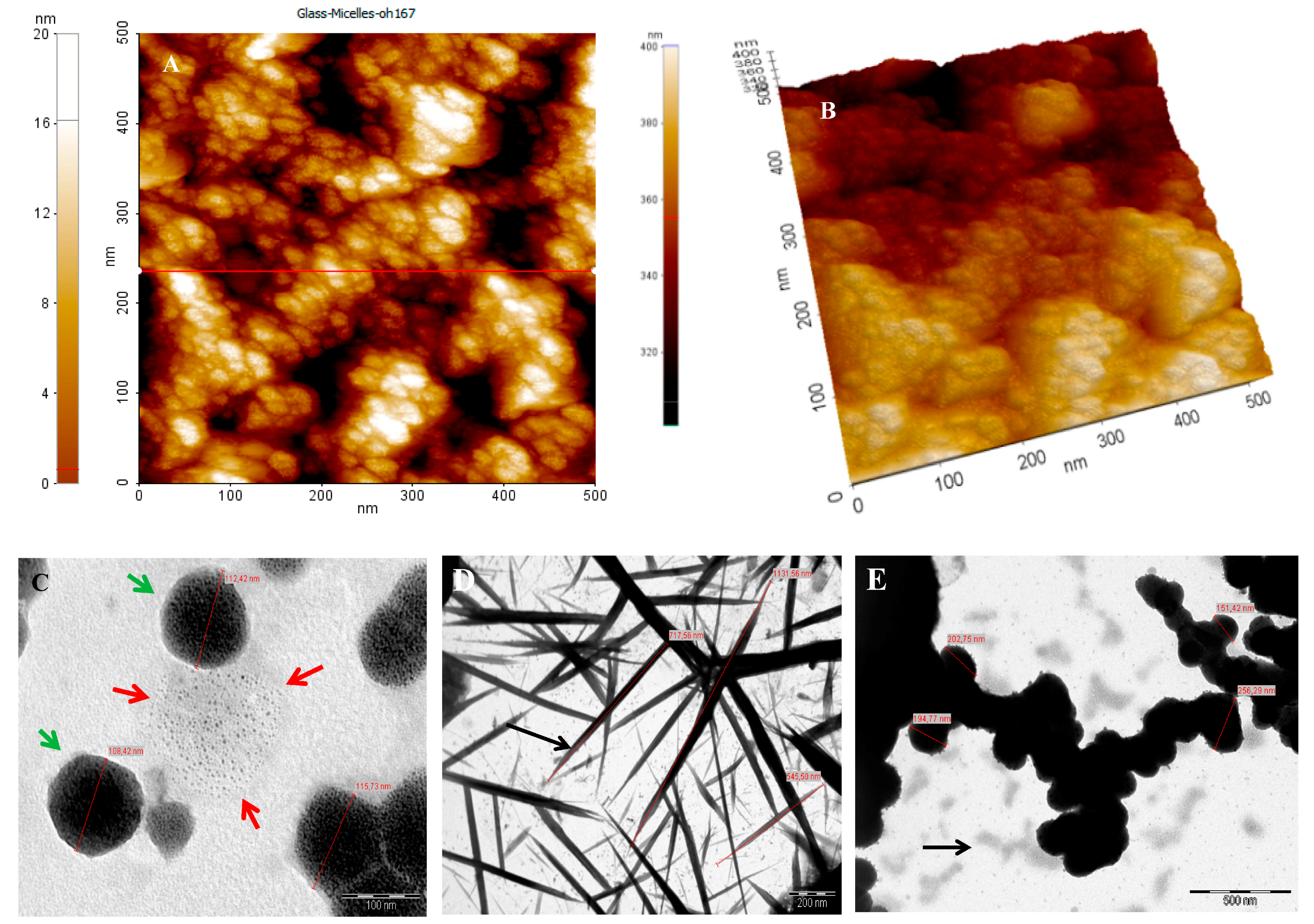
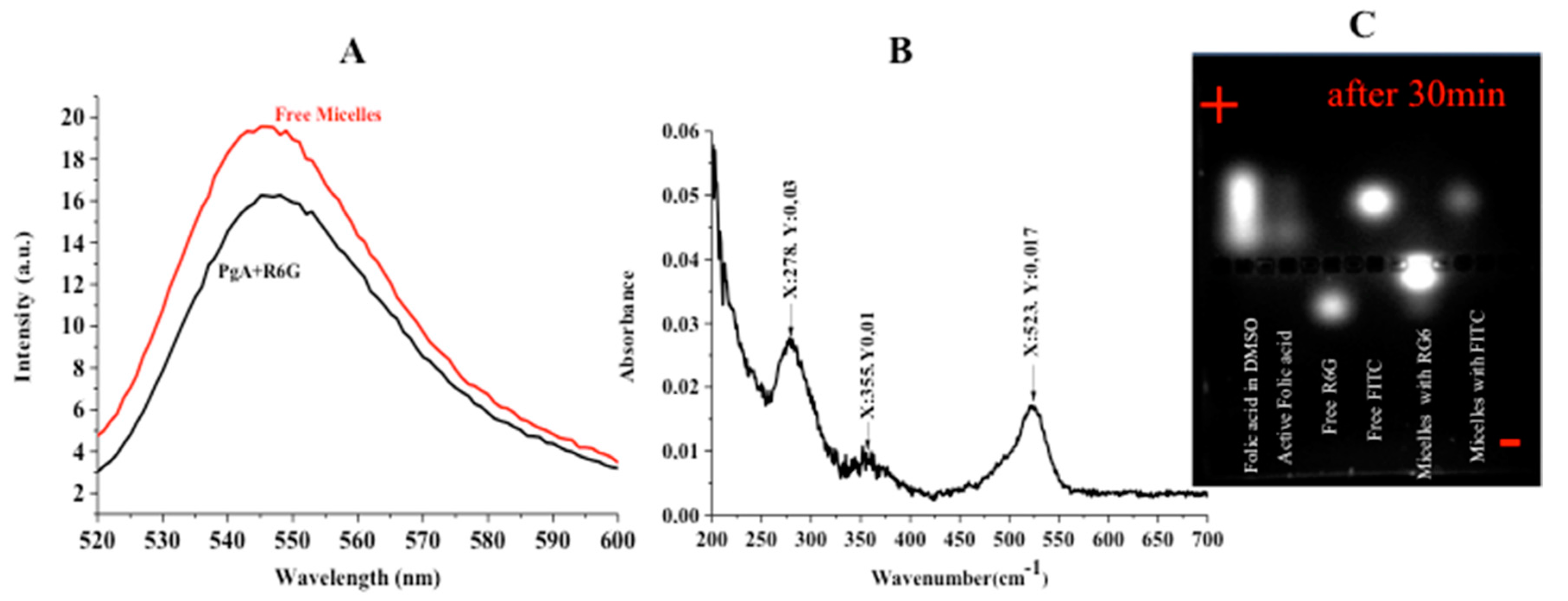
2.4. Characterization
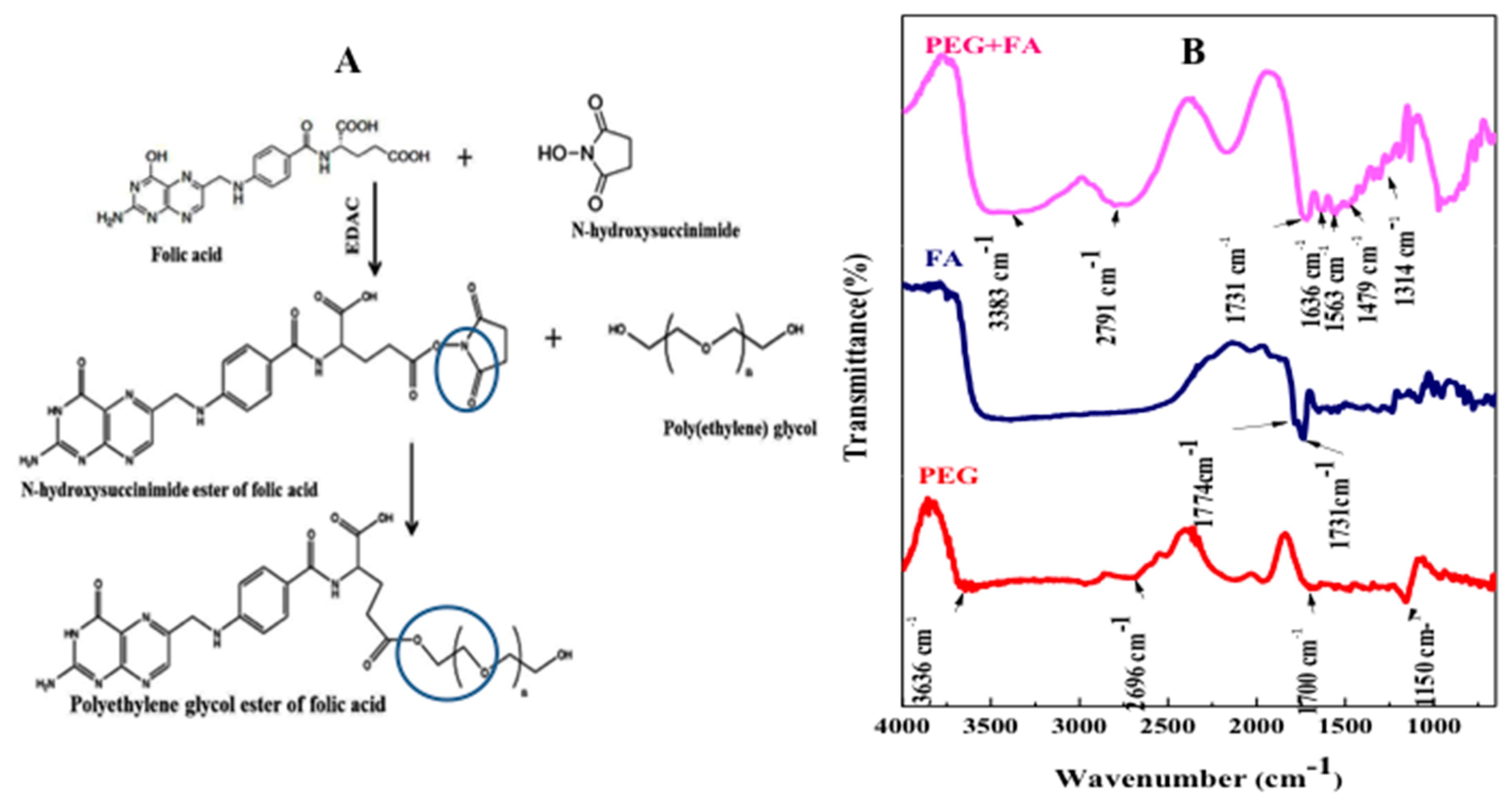
2.5. FTIR Characterization, Folic Acid Conjugation and Structure Assembly
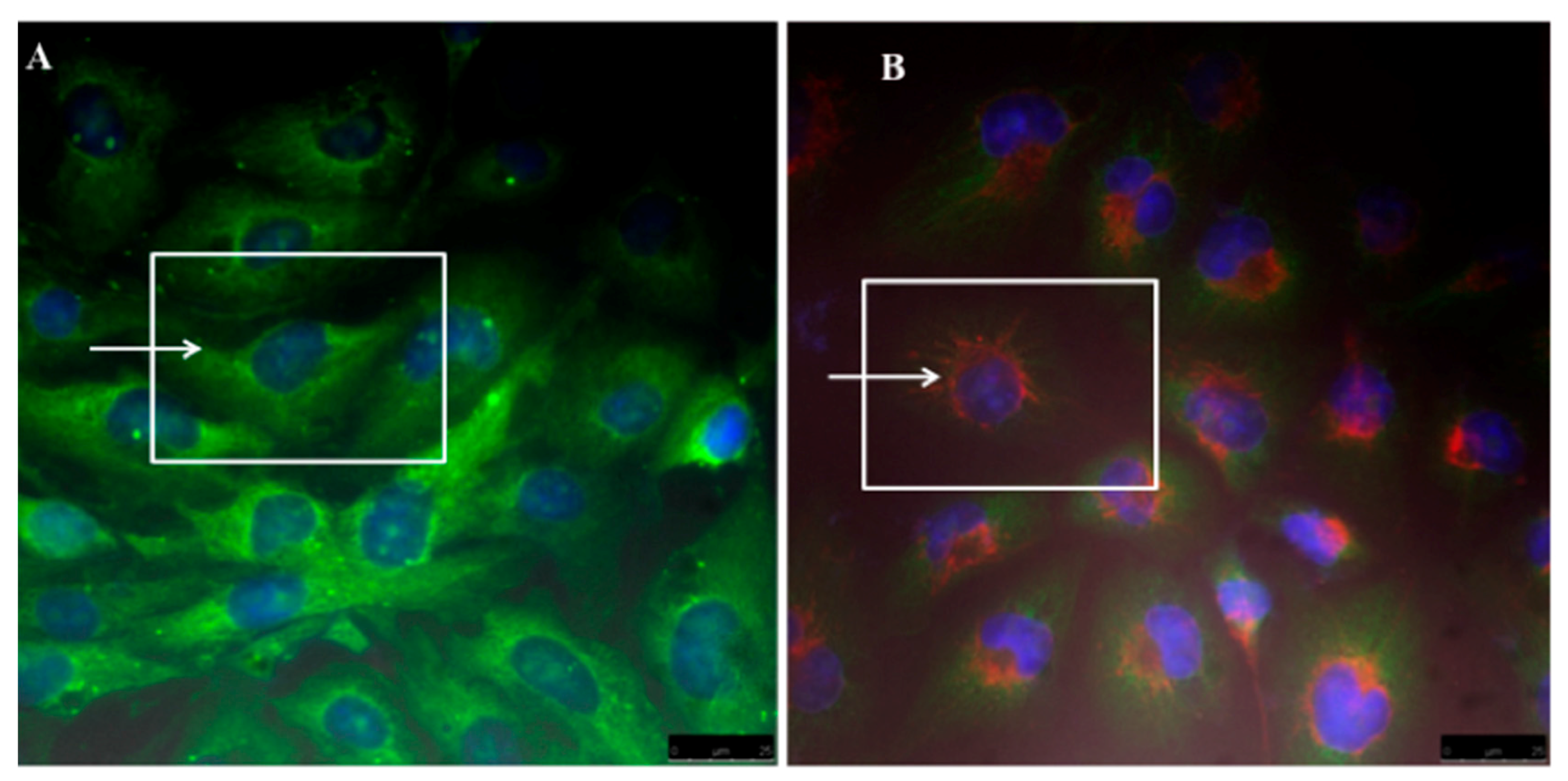
2.6. Cell Cultures and Micelles Uptake
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Fabrication of Nano-Micelles
4.3. Transmission Electron Microscopy (TEM)
4.4. Atomic Force Microscopy (AFM)
4.5. Absorbance and Fluorescence Spectrophotometry
4.6. Fourier Transform Infrared Spectroscopy (FTIR)
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chow, E.K.; Ho, D. Cancer Nanomedicine: From Drug Delivery to Imaging. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Burt, H.M.; Mangold, G.; Dexter, D.; Von Hoff, D.; Mayer, L.; Hunter, W.L. Anti-tumour efficacy and bio-distribution of intravenous polymeric micellar paclitaxel. Anticancer Drugs 1997, 8, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.G.; Kim, S.Y.; Lee, Y.M.; Cho, C.S.; Sung, Y.K. Methoxy Poly(ehylene glycol)/ε-Caprolactone Amphiphilic Block Copolymeric Micelle containing Indomethacin. I. Preparation and characterization. J. Control Release 1998, 51, 1–11. [Google Scholar] [CrossRef]
- Yu, B.G.; Okano, T.; Kataoka, K.; Sardari, S.; Kwon, G.S. In vitro dissociation of antifungal efficacy and toxicity for amphotericin B-loaded poly(ethylene oxide)-block-poly(beta-benzyl-l-aspartate) micelles. J. Control Release 1998, 56, 285–291. [Google Scholar] [CrossRef]
- Jeong, Y.L.; Nah, J.W.; Lee, H.C.; Kim, S.H.; Cho, C.S. Adriamycin release from flower-type polymeric micelle based on star-block copolymer composed of poly(gamma-benzyl-l-glutamate) as the hydrophobic part and poly(ethylene oxide) as the hydrophilic part. Int. J. Pharm. 1999, 188, 49–58. [Google Scholar] [CrossRef]
- Hanafy, N.A.; Dini, L.; Citti, C.; Cannazza, G.; Leporatti, S. Inhibition of Glycolysis by Using a Micro/Nano-Lipid Bromopyruvic Chitosan Carrier as a Promising Tool to Improve Treatment of Hepatocellular Carcinoma. Nanomaterials 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Kataoka, K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006, 112, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N.; Quarta, A.; Di Corato, R.; Dini, L.; Nobile, C.; Tasco, V.; Carallo, S.; Cascione, M.; Malfettone, A.; Soukupova, J.; et al. Hybrid polymeric-protein nano-carriers (HPPNC) for targeted delivery of TGFβ inhibitors to hepatocellular carcinoma cells. J. Mater. Sci. Mater. Med. 2017, 28, 120. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur. J. Pharm. Biopharm. 2009, 71, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Welsh, W.J.; Moghe, P.V.; Uhrich, K.E. Micellar and structural stability of nanoscale amphiphilic polymers: Implications for anti-atherosclerotic bioactivity. Biomaterials 2016, 84, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef]
- Gueorguieva, I.; Cleverly, A.L.; Stauber, A.; Sada Pillay, N.; Rodon, J.A.; Miles, C.P.; Yingling, J.M.; Lahn, M.M. Defining a therapeutic window for the novel TGF-β inhibitor LY2157299 monohydrate based on a pharmacokinetic/pharmacodynamic model. Br. J. Clin. Pharmacol. 2014, 77, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.; Ferraro, M.M.; Gaballo, A.; Dini, L.; Tasco, V.; Nobile, C.; De Giorgi, M.L.; Carallo, S.; Rinaldi, R.; Leporatti, S. Fabrication and Characterization of ALK1fc-Loaded Fluoro-Magnetic Nanoparticles Rods for Inhibiting TGF β1 in HCC. RSC Adv. 2016, 6, 48834–48842. [Google Scholar] [CrossRef]
- Bueno, L.; de Alwis, D.P.; Pitou, C.; Yingling, J.; Lahn, M.; Glatt, S.; Trocóniz, I.F. Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur. J. Cancer 2008, 44, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Taipale, J.; Miyazono, K.; Heldin, C.H.; Keski-Oja, J. Latent transforming growth factor beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J. Cell Biol. 1994, 124, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.L.; Jagadeeshan, S.; Nair, S.A.; Kumar, G.S.V. Folic Acid Conjugated δ-Valerolactone-Poly(ethylene glycol) Based Triblock Copolymer as a Promising Carrier for Targeted Doxorubicin Delivery. PLoS ONE 2013, 8, e70697. [Google Scholar] [CrossRef] [PubMed]
- Ralet, M.C.; Dronnet, V.; Buchholt, H.C.; Thibault, J.F. Enzymatically and chemically de-esterified lime pectins: Characterisation, polyelectrolyte behaviour and calcium binding properties. Carbohydr. Res. 2001, 336, 117–125. [Google Scholar] [CrossRef]
- Tolentino Chivite, A. Ionic Complexes of Biodegradable Polyelectrolytes. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2014. [Google Scholar]
- Abdelaal, M.Y.; Makki, M.S.I.; Sobahi Tariq, R.A. Modification and Characterization of Polyacrylic Acid for Metal Ion Recovery. Am. J. Polym. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Fang, X.; Somasundaran, P. Swelling of Poly(acrylic acid) in Concentrated Sodium Carbonate Solution. J. Chem. Eng. Data 2010, 55, 3555–3559. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Kavimandan, N.J. Nanoscale analysis of protein and peptide absorption: Insulin absorption using complexation and pH-sensitive hydrogels as delivery vehicles. Eur. J. Pharm. Sci. 2006, 29, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Mundargi, R.C.; Rangaswamy, V.; Aminabhavi, T.M. pH-Sensitive oral insulin delivery systems using Eudragit microspheres. Drug Dev. Ind. Pharm. 2011, 37, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Wood, K.M.; Blanchette, J.O. Hydrogels for oral delivery of therapeutic proteins. Expert Opin. Biol. Ther. 2004, 4, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Alhaique, F.; Santucci, E.; Carafa, M.; Coviello, T.; Murtas, E.; Riccieri, F.M. Gellan in sustained release formulations: Preparation of gel capsules and release studies. Biomaterials 1996, 17, 1981–1986. [Google Scholar] [CrossRef]
- Serra, L.; Doménech, J.; Peppas, N.A. Engineering design and molecular dynamics of mucoadhesive drug delivery systems as targeting agents. Eur. J. Pharm. Biopharm. 2009, 71, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Kost, J. Intelligent drug delivery systems. In Encyclopaedia of Controlled Drug Delivery; Mathiowitz, E., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1999; pp. 445–459. [Google Scholar]
- Bastakotin, B.P.; Liao, S.-H.; Inoue, M.; Yusa, S.-I.; Imura, M.; Nakashima, K.; Wu, K.C.; Yamauchi, Y. pH-responsive polymeric micelles with Core-shell corona architectures as intracellular anti-cancer drug carriers. Sci. Technol. Adv. Mater. 2013, 14, 044402. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, J.S.; Mo, Y.; Tan, R.X. Calcium pectinate capsules for colon-specific drug delivery. Drug Dev. Ind. Pharm. 2005, 31, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.E.; Macdonald, M.; Nie, J.; Bowman, C.N. Structure and swelling of poly(acrylic acid) hydrogels: Effect of pH, ionic strength, and dilution on the cross-linked polymer structure. Polymer 2004, 45, 1503–1510. [Google Scholar] [CrossRef]
- Itoh, K.; Kubo, W.; Fujiwara, M.; Watanabe, H.; Miyazaki, S.; Attwood, D. The influence of gastric acidity and taste masking agent on in situ gelling pectin formulations for oral sustained delivery of acetaminophen. Biol. Pharm. Bull. 2006, 29, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Bromberg, P.R.; Hatton, T.A.; Tam, K.C. Synthesis and aggregation behaviour of pluronic f87/polyacrylic acid block copolymer in the presence of doxorubicin. Langmuir 2007, 23, 2638–2646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Eisenberg, A. Multiple morphologies of “crew-cut” aggregates of polystyrene-b-poly(acrylic acid) block copolymers. Science 1995, 268, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Iatridi, Z.; Tsitsilianis, C. Water-Soluble Stimuli Responsive Star-Shape Segmented Macromolecules. Polymers 2011, 3, 1911–1933. [Google Scholar] [CrossRef]
- Pan, D.; Turner, J.L.; Wooley, K.L. Folic acid-conjugated nanostructured materials designed for cancer cell targeting. Chem. Commun. 2003, 7, 2400–2401. [Google Scholar] [CrossRef]
- Lee, J.G.; Kay, E.P. FGF-2-induced wound healing in corneal endothelial cells requires Cdc42 activation and Rho inactivation through the phosphatidylinositol 3-kinase pathway. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Laing, B.M.; Guo, P.; Bergstrom, D.E. Optimized method for the synthesis and purification of adenosine—Folic acid conjugates for use as transcription initiators in the preparation of modified RNA. Methods 2011, 54, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.S.; Naval, A.; Patil, R.; Khandare, J. Poly(ethylene glycol)-Prodrug Conjugates: Concept, Design, and Applications. J. Drug Deliv. 2012, 2012, 103973. [Google Scholar] [CrossRef] [PubMed]
- Khoee, S.; Kavand, A. A new procedure for preparation of polyethylene glycol-grafted magnetic iron oxide nanoparticles. J. Nanostruct. Chem. 2014, 4, 111. [Google Scholar] [CrossRef]
- Sánchez-Márquez, A.; Fuentes-Ramírez, R.; Cano-Rodríguez, I.; Gamiño-Arroyo, Z.; Rubio-Rosas, E.; Kenny, J.M.; Rescignano, N. Membrane Made of Cellulose Acetate with Polyacrylic Acid Reinforced with Carbon Nanotubes and Its Applicability for Chromium Removal. Int. J. Polym. Sci. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Manrique, G.D.; Lajolo, F.M. FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Postharvest Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Li, J.; Chen, T.; Deng, F.; Wan, J.; Tang, Y.; Yuan, P.; Zhang, L. Synthesis, characterization, and in vitro evaluation of curcumin-loaded albumin nanoparticles surface-functionalized with glycyrrhetinic acid. Int. J. Nanomed. 2015, 10, 5475–5487. [Google Scholar] [CrossRef]
- Kato, T.; Matsuoka, T.; Nishii, M.; Kamikawa, Y.; Kanie, K.; Nishimura, T.; Yashima, E.; Ujiie, S. Supramolecular chirality of thermotropic liquid-crystalline folic acid derivatives. Angew. Chem. Int. Ed. Engl. 2004, 43, 1969–1972. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanafy, N.A.N.; Quarta, A.; Ferraro, M.M.; Dini, L.; Nobile, C.; De Giorgi, M.L.; Carallo, S.; Citti, C.; Gaballo, A.; Cannazza, G.; et al. Polymeric Nano-Micelles as Novel Cargo-Carriers for LY2157299 Liver Cancer Cells Delivery. Int. J. Mol. Sci. 2018, 19, 748. https://doi.org/10.3390/ijms19030748
Hanafy NAN, Quarta A, Ferraro MM, Dini L, Nobile C, De Giorgi ML, Carallo S, Citti C, Gaballo A, Cannazza G, et al. Polymeric Nano-Micelles as Novel Cargo-Carriers for LY2157299 Liver Cancer Cells Delivery. International Journal of Molecular Sciences. 2018; 19(3):748. https://doi.org/10.3390/ijms19030748
Chicago/Turabian StyleHanafy, Nemany Abdelhamid Nemany, Alessandra Quarta, Marzia Maria Ferraro, Luciana Dini, Concetta Nobile, Maria Luisa De Giorgi, Sonia Carallo, Cinzia Citti, Antonio Gaballo, Giuseppe Cannazza, and et al. 2018. "Polymeric Nano-Micelles as Novel Cargo-Carriers for LY2157299 Liver Cancer Cells Delivery" International Journal of Molecular Sciences 19, no. 3: 748. https://doi.org/10.3390/ijms19030748