Diazoxide Improves Mitochondrial Connexin 43 Expression in a Mouse Model of Doxorubicin-Induced Cardiotoxicity
Abstract
:1. Introduction
2. Results
2.1. Cardiac Functions
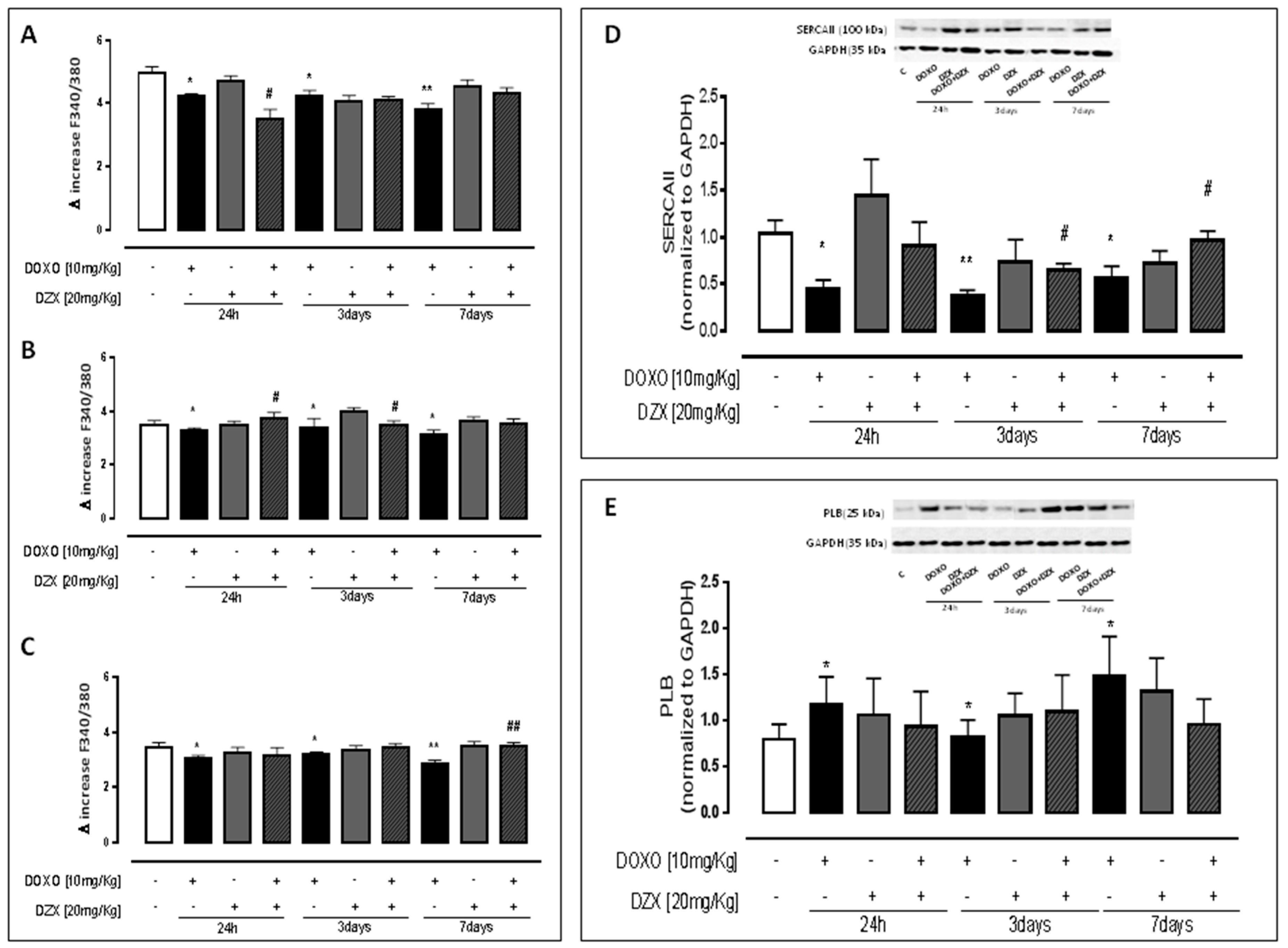
2.2. Diazoxide Administration Alters Calcium Homeostasis
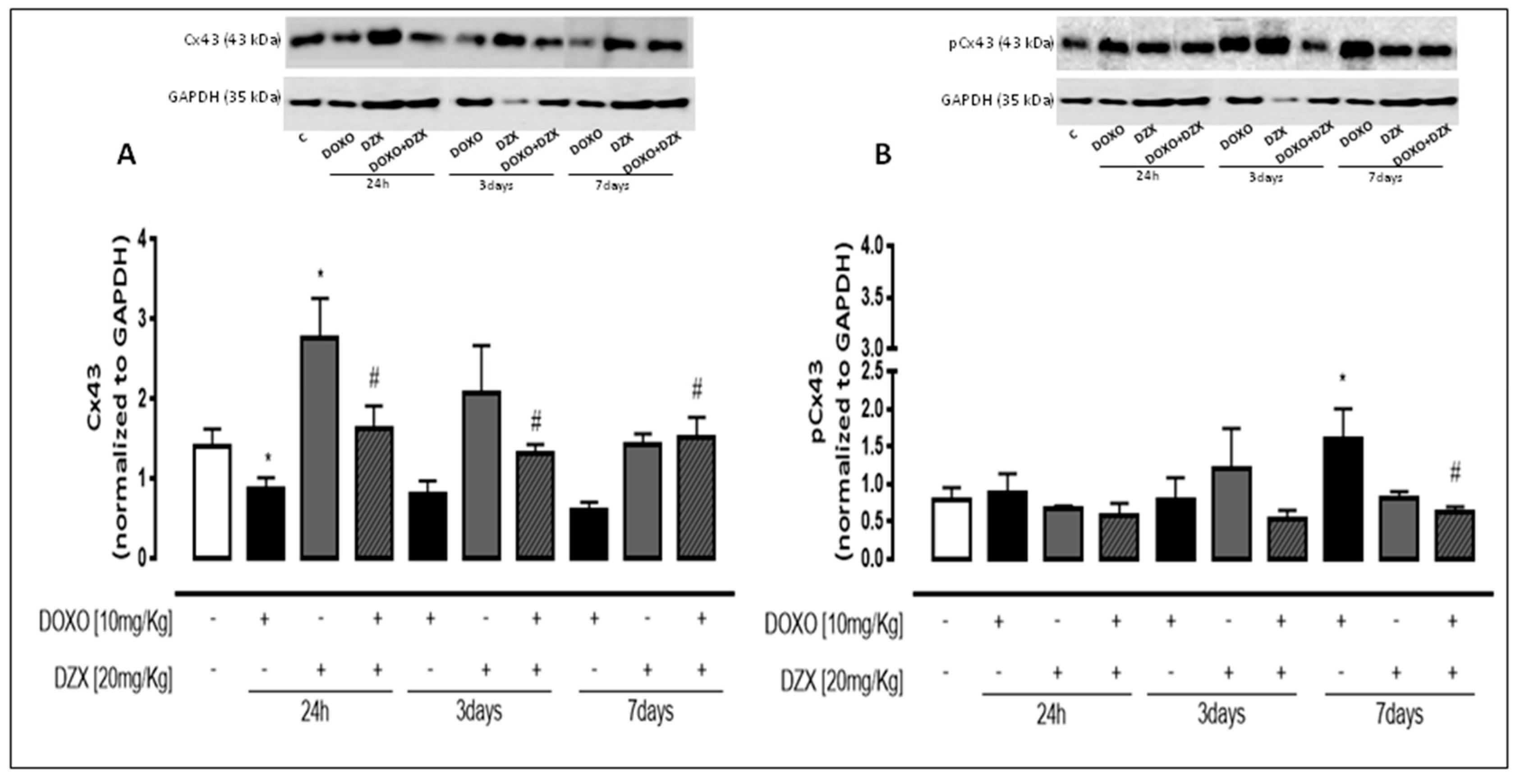
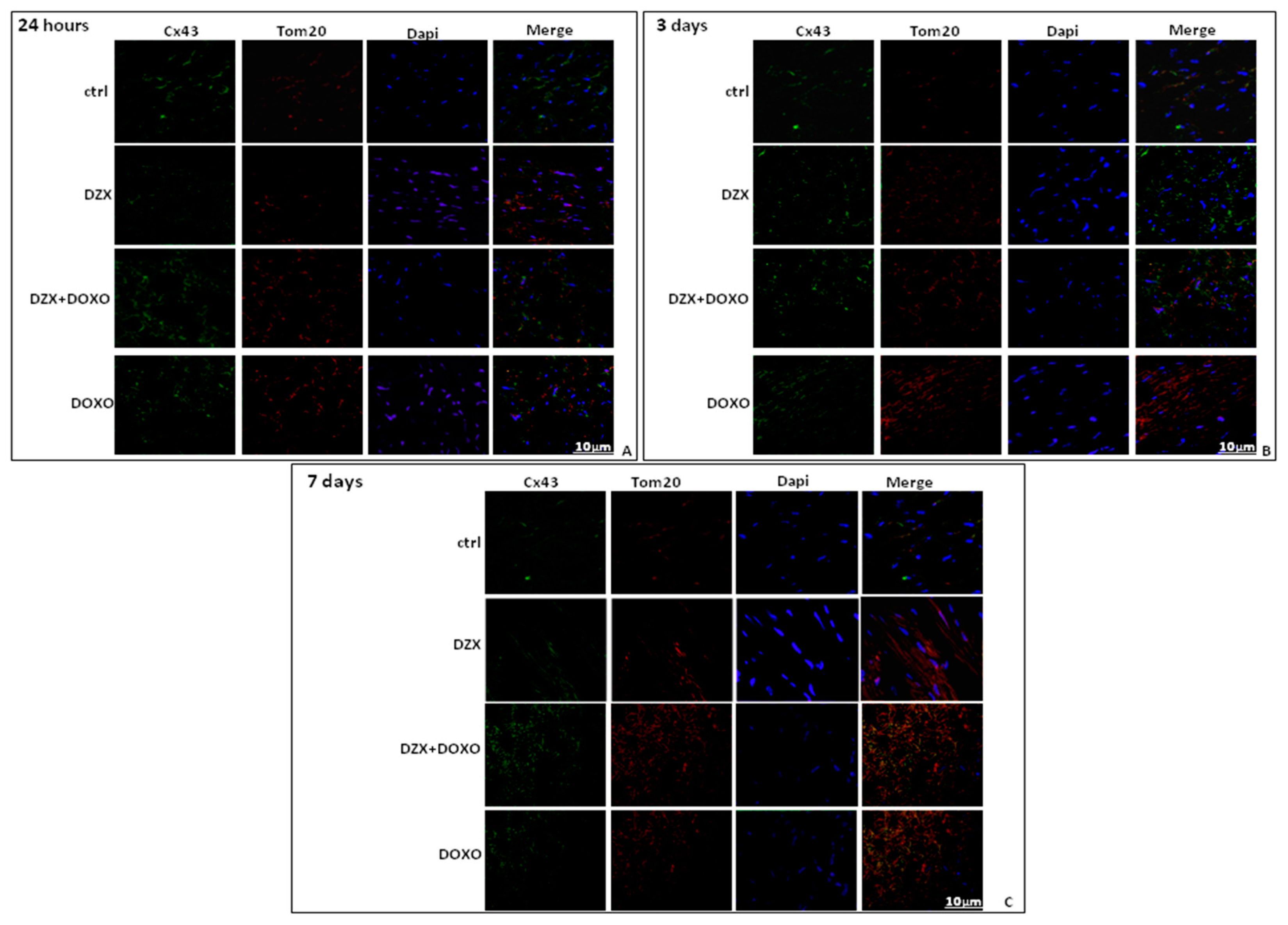
2.3. Diazoxide Administration Affects Cx43 and pCx43 Expression and Localization
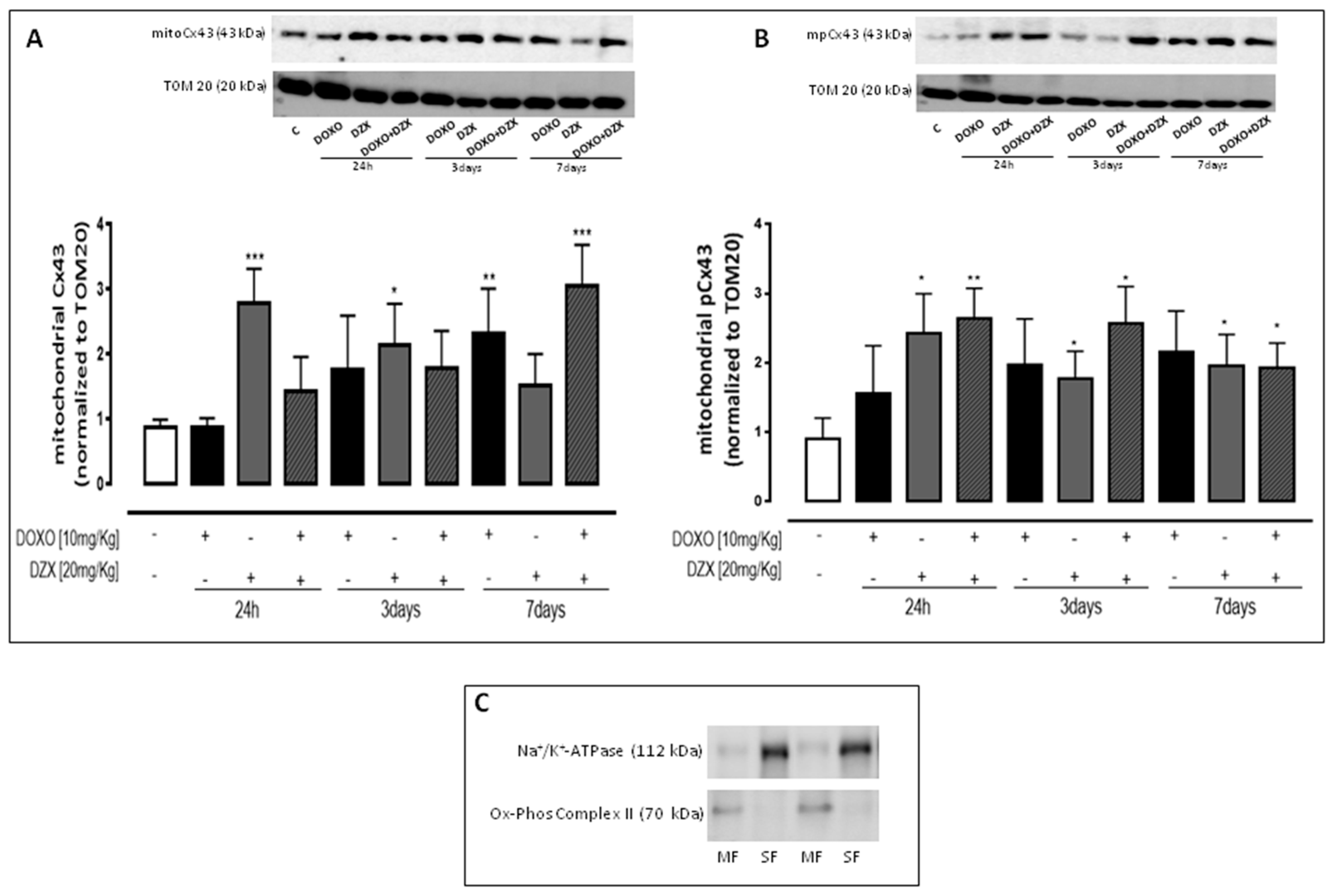
2.4. Diazoxide Administration Affects the Mitochondrial Amount of Cx43 and pCx43
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Experimental Protocols
- 3 mice received a single DOXO administration (10 mg/kg i.p.) and were sacrificed 24 h after the treatment.
- 3 mice received a single DZX administration (20 mg/kg i.p.) and were sacrificed 24 h after the treatment.
- 3 mice were pretreated with DZX (20 mg/kg i.p.) and then received a single DOXO (10 mg/kg i.p) administration and were sacrificed 24 h after the treatment.
- 3 mice received DOXO (10 mg/kg i.p.) every other day and were sacrificed 3 days after the treatment.
- 3 mice received DZX (20 mg/kg i.p.) every other day and were sacrificed 3 days after the treatment.
- 3 mice were pretreated with DZX (20 mg/kg i.p.) and then received DOXO (10 mg/kg i.p.) every other day and were sacrificed 3 days after the treatment.
- 3 mice received DOXO (10 mg/kg i.p.) every other day and were sacrificed 7 days after the treatment.
- 3 mice received DZX (20 mg/kg i.p.) every other day and were sacrificed 7 days after the treatment.
- 3 mice were pretreated with DZX (20 mg/kg i.p.) and then received DOXO (10 mg/kg i.p.) every other day and were sacrificed 7 days after the treatment.
4.4. Echocardiogram
4.5. Protein Extraction and Western Blot Analysis
4.6. Mitochondrial Protein Extraction and Western Blot Analysis for Mitochondrial Cx43 and pCx43
4.7. Primary Cardiomyocytes Isolation and Measurement of Intracellular Ca2+ Signaling
4.8. Immunohistochemical Analysis
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hrdina, R.; Gersl, V.; Klimtová, I.; Simůnek, T.; Machácková, J.; Adamcová, M. Anthracycline-induced cardiotoxicity. Acta Med. (Hrad. Kralove) 2000, 43, 75–82. [Google Scholar]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Smith, L.B.; Magun, E.A.; Engstrom, T.; Kelley-Howard, K.; Jandhyala, D.M.; Thorpe, C.M.; Magun, B.E.; Wood, L.J. Small molecule kinase inhibitors block the ZAK-dependent inflammatory effects of doxorubicin. Cancer Biol. Ther. 2013, 14, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, K.K.; Wallace, K.B. Keap1 redox-dependent regulation of doxorubicin-induced oxidative stress response in cardiac myoblasts. Toxicol. Appl. Pharmacol. 2014, 274, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Kavazis, A.N.; Morton, A.B.; Hall, S.E.; Smuder, A.J. Effects of doxorubicin on cardiac muscle subsarcolemmal and intermyofibrillar mitochondria. Mitochondrion 2016, 34, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ghigo, A.; Li, M.; Hirsch, E. New signal transduction paradigms in anthracycline-induced cardiotoxicity. Biochim. Biophys. Acta 2016, 1863, 1916–1925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Portera, C.C.; Swain, S.M. The heart of the matter. J. Clin. Oncol. 2007, 25, 3794–3796. [Google Scholar] [CrossRef] [PubMed]
- Menna, P.; Salvatorelli, E.; Minotti, G. Cardiotoxicity of antitumor drugs. Chem. Res. Toxicol. 2008, 21, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Del Pizzo, M.; Marzocco, S.; Sorrentino, R.; Ciccarelli, M.; Iaccarino, G.; Pinto, A.; Popolo, A. Inflammatory mediators in a short-time mouse model of doxorubicin-induced cardiotoxicity. Toxicol. Appl. Pharmacol. 2016, 293, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Rodríguez-Sinovas, A.; Marzocco, S.; Ciccarelli, M.; Iaccarino, G.; Pinto, A.; Popolo, A. Cardiotoxic Effects of Short-Term Doxorubicin Administration: Involvement of Connexin 43 in Calcium Impairment. Int. J. Mol. Sci. 2017, 18, 2121. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Verrilli, V.; Pinto, A.; Popolo, A. Role of connexin 43 in cardiovascular diseases. Eur. J. Pharmacol. 2015, 768, 71–76. [Google Scholar]
- Miro-Casas, E.; Ruiz-Meana, M.; Agullo, E.; Stahlhofen, S.; Rodríguez-Sinovas, A.; Cabestrero, A.; Jorge, I.; Torre, I.; Vazquez, J.; Boengler, K.; et al. Connexin43 in cardiomyocyte mitochondria contributes to mitochondrial potassium uptake. Cardiovasc. Res. 2009, 83, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Ruiz-Meana, M.; Gent, S.; Ungefug, E.; Soetkamp, D.; Miro-Casas, E.; Cabestrero, A.; Fernandez-Sanz, C.; Semenzato, M.; Di Lisa, F.; et al. Mitochondrial connexin 43 impacts on respiratorycomplex I activity and mitochondrial oxygenconsumption. J. Cell. Mol. Med. 2012, 16, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Gadicherla, A.K.; Wang, N.; Bulic, M.; Agullo-Pascual, E.; Lissoni, A.; De Smet, M.; Delmar, M.; Bultynck, G.; Krysko, D.V.; Camara, A.; et al. Mitochondrial Cx43 hemichannels contribute to mitochondrial calcium entry and cell death in the heart. Basic Res. Cardiol. 2017, 112, 27. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Sorrentino, R.; Franceschelli, S.; Del Pizzo, M.; Pinto, A.; Popolo, A. Doxorubicin-Mediated Cardiotoxicity: Role of Mitochondrial Connexin 43. Cardiovasc. Toxicol. 2015, 15, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Garlid, K.D.; Dos Santos, P.; Xie, Z.J.; Costa, A.D.; Paucek, P. Mitochondrial potassium transport: The role of the mitochondrial ATP-sensitive K(+) channel in cardiac function and cardioprotection. Biochim. Biophys. Acta 2003, 1606, 1–21. [Google Scholar] [CrossRef]
- Nichols, C.G.; Singh, G.K.; Grange, D.K. KATP channels and cardiovascular disease: Suddenly a syndrome. Circ. Res. 2013, 112, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Noma, A. ATP-regulated K+ channels in cardiac muscle. Nature 1983, 305, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Henn, M.C.; Janjua, M.B.; Zhang, H.; Kanter, E.M.; Makepeace, C.M.; Schuessler, R.B.; Nichols, C.G.; Lawton, J.S. Diazoxide Cardioprotection Is Independent of Adenosine Triphosphate-Sensitive Potassium Channel Kir6.1 Subunit in Response to Stress. J. Am. Coll. Surg. 2015, 2, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, M.; Bhupathy, P.; Babu, G.J. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc. Res. 2008, 77, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Hood, A.R.; Ai, X.; Pogwizd, S.M. Regulation of cardiac gap junctions by protein phosphatases. J. Mol. Cell. Cardiol. 2017, 107, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, W.J.; Li, Y.J.; Wei, L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch. Immunol. Ther. Exp. (Warsz) 2009, 57, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Singal, P.K.; Li, T.; Kumar, D.; Danelisen, I.; Iliskovic, N. Adriamycin-induced heart failure: Mechanism and modulation. Mol. Cell. Biochem. 2000, 207, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ueda, Y.; Juan, Y.; Katsuda, S.; Takahashi, H.; Koh, E. Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: In vivo study. Circulation 2000, 102, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Severs, N.J.; Bruce, A.F.; Dupont, E.; Rothery, S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc. Res. 2008, 80, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, W.A. Multiplicity of effectors of the cardioprotective agent, diazoxide. Pharmacol. Ther. 2013, 140, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Cabo, C.; Boyden, P.A. Extracellular space attenuates the effect of gap junctional remodeling on wave propagation: A computational study. Biophys. J. 2009, 96, 3092–3101. [Google Scholar] [CrossRef] [PubMed]
- Facundo, H.T.F.; de Paula, J.G.; Kowaltowski, A.J. Mitochondrial ATP-sensitive K+ channels prevent oxidative stress, permeability transition and cell death. J. Bioenerg. Biomembr. 2005, 37, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.; Zaldivar, D.; Carrillo, E.; Hernandez, A.; Garcia, M.; Sanchez, J. Pharmacological preconditioning by diazoxide downregulates cardiac l-type Ca(2+). Br. J. Pharmacol. 2010, 161, 1172–1185. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Crisosto, C.; Pennanen, C.; Vasquez-Trincado, C.; Morales, P.E.; Bravo-Sagua, R.; Quest, A.F.G.; Chiong, M.; Lavandero, S. Sarcoplasmic reticulum-mitochondria communication in cardiovascular pathophysiology. Nat. Rev. Cardiol. 2017, 14, 342–360. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Wu, Z.; Li, X.; Yan, J.; Zhao, L.; Yang, C.; Lu, J.; Deng, J.; Chen, M. Resveratrol ameliorates cardiac dysfunction induced by pressure overload in rats via structural protection and modulation of Ca(2+) cycling proteins. J. Transl. Med. 2014, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Marks, A.R. Calcium cycling proteins and heart failure: Mechanisms and therapeutics. J. Clin. Investig. 2013, 123, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Zhang, M.; Tang, Y.; Xie, Y.; Huang, X.; Li, Y. Doxorubicin induces sarcoplasmic reticulum calcium regulation dysfunction via the decrease of SERCA2 and phospholamban expressions in rats. Cell Biochem. Biophys. 2014, 70, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.M.; Caldas, F.R.; da Silva, A.P.; Ventura, M.M.; Leite, I.M.; Filgueiras, A.B.; Silva, C.G.; Kowaltowski, A.J.; Facundo, H.T. Diazoxide prevents reactive oxygen species and mitochondrial damage, leading to anti-hypertrophic effects. Chem. Biol. Interact. 2017, 261, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dorado, D.; Ruiz-Meana, M.; Inserte, J.; Rodriguez-Sinovas, A.; Piper, H. Calcium-mediated cell death during myocardial reperfusion. Cardiovasc. Res. 2012, 94, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Shen, P.P.; Zhao, M.M.; Liu, X.P.; Xie, H.Y.; Deng, F.; Feng, J.C. Mechanism of mitochondrial Connexin43’s protection of the neurovascular unit under acute cerebral ischemia-repurfusion injury. Int. J. Mol. Sci. 2016, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, N.; Zhao, Z.; Han, F.; Wang, X.; Zeng, Y. Ischemic postconditioning improves the expression of cellular membrane connexin 43 and attenuates the reperfusion injury in rat acute myocardial infarction. Biomed. Rep. 2015, 3, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Lee, S.C.; Reuss, L.; Altenberg, G.A. Change in permeant size selectivity by phosphorylation of connexin 43 gap-junctional hemichannels by PKC. Proc. Natl. Acad. Sci. USA 2007, 104, 4919–4924. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Kim, M.J.; Yoon, I.S.; Ahn, J.H.; Lee, S.H.; Baik, E.J.; Moon, C.H.; Jung, Y.S. Diazoxide acts more as a PKC-epsilon activator, and indirectly activates the mitochondrial K(ATP) channel conferring cardioprotection against hypoxic injury. Br. J. Pharmacol. 2006, 149, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.; Lin, D.; Akoyev, V.; Willard, L.; Takemoto, D. Protein Kinase C Epsilon Activates Lens Mitochondrial Cytochrome C Oxidase Subunit IV During Hypoxia. Exp. Eye Res. 2008, 86, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.K.; Cheng, H.H.; Wang, S.D.; Yeih, D.F.; Wang, S.M. PKCɛ mediates serine phosphorylation of connexin43 induced by lysophosphatidylcholine in neonatal rat cardiomyocytes. Toxicology 2013, 314, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Lampe, P.D.; Lau, A.F. Regulation of gap junctions by phosphorylation of connexins. Arch. Biochem. Biophys. 2000, 384, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, A.; Zolfaghari, S.; Ostadhadi, S. Anticonvulsant effect of Diazoxide against Dichlorvos-induced seizures in mice. Sci. World J. 2013, 2013, 697305. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Dodoni, G.; Rodriguez-Sinovas, A.; Cabestrero, A.; Ruiz-Meana, M.; Gres, P.; Konietzka, I.; Lopez-Iglesias, C.; Garcia-Dorado, D.; Di Lisa, F.; et al. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc. Res. 2005, 67, 234–244. [Google Scholar] [CrossRef] [PubMed]
| 24 h | Control | DOXO | DZX | DZX+DOXO | |
| LVEDD | 3.97 ± 0.11 | 4.09 ± 0.1 | 3.8 ± 0.010 | 3.725 ± 0.145 | |
| LVESD | 2.62 ± 0.17 | 3.00 ± 0.06 * | 2.8 ± 0.40 | 2.67 ± 0.146 | |
| %EF | 62.17 ± 4.1 | 52.7 ± 1.38 ** | 53.0 ± 5.0 | 54.25 ± 1.8 # | |
| %FS | 30.41 ± 0.72 | 26.6 ± 0.92 * | 27.0 ± 3.000 | 27.5 ± 0.936 # | |
| 3 days | Control | DOXO | DZX | DZX+DOXO | |
| LVEDD | 3.940 ± 0.050 | 3.96 ± 0.06 | 3.895 ± 0.005 | 3.792 ± 0.086 | |
| LVESD | 2.780 ± 0.054 | 2.90 ± 0.056 * | 2.70 ± 0.400 | 2.823 ± 0.107 | |
| %EF | 57.20 ± 1.25 | 53.73 ± 1.61 * | 70.0 ± 1.000 | 50.500 ± 2.217 | |
| %FS | 30.410 ± 0.850 | 27.43 ± 1.02 * | 39.5 ± 0.500 | 25.000 ± 0.354 # | |
| 7 days | Control | DOXO | DZX | DZX+DOXO | |
| LVEDD | 3.860 ± 0.040 | 3.96 ± 0.055 * | 3.515 ± 0.055 | 3.710 ± 0.079 | |
| LVESD | 2.730 ± 0.150 | 2.9 ± 0.14 | 2.350 ± 0.350 | 2.553 ± 0.135 | |
| %EF | 59.000 ± 4.170 | 50.49 ± 4.79 ** | 63.0 ± 2.000 | 57.00 ± 2.606 # | |
| %FS | 31.760 ± 1.680 | 25.39 ± 3.02 * | 32.00 ± 2.000 | 29.000 ± 1.517 # |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecoraro, M.; Ciccarelli, M.; Fiordelisi, A.; Iaccarino, G.; Pinto, A.; Popolo, A. Diazoxide Improves Mitochondrial Connexin 43 Expression in a Mouse Model of Doxorubicin-Induced Cardiotoxicity. Int. J. Mol. Sci. 2018, 19, 757. https://doi.org/10.3390/ijms19030757
Pecoraro M, Ciccarelli M, Fiordelisi A, Iaccarino G, Pinto A, Popolo A. Diazoxide Improves Mitochondrial Connexin 43 Expression in a Mouse Model of Doxorubicin-Induced Cardiotoxicity. International Journal of Molecular Sciences. 2018; 19(3):757. https://doi.org/10.3390/ijms19030757
Chicago/Turabian StylePecoraro, Michela, Michele Ciccarelli, Antonella Fiordelisi, Guido Iaccarino, Aldo Pinto, and Ada Popolo. 2018. "Diazoxide Improves Mitochondrial Connexin 43 Expression in a Mouse Model of Doxorubicin-Induced Cardiotoxicity" International Journal of Molecular Sciences 19, no. 3: 757. https://doi.org/10.3390/ijms19030757





