Suppression Effect of Astaxanthin on Osteoclast Formation In Vitro and Bone Loss In Vivo
Abstract
:1. Introduction
2. Results
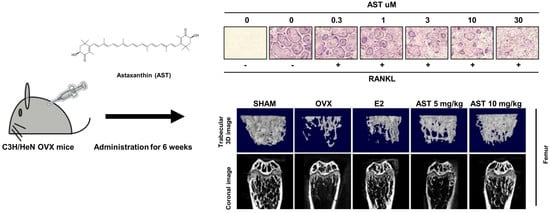
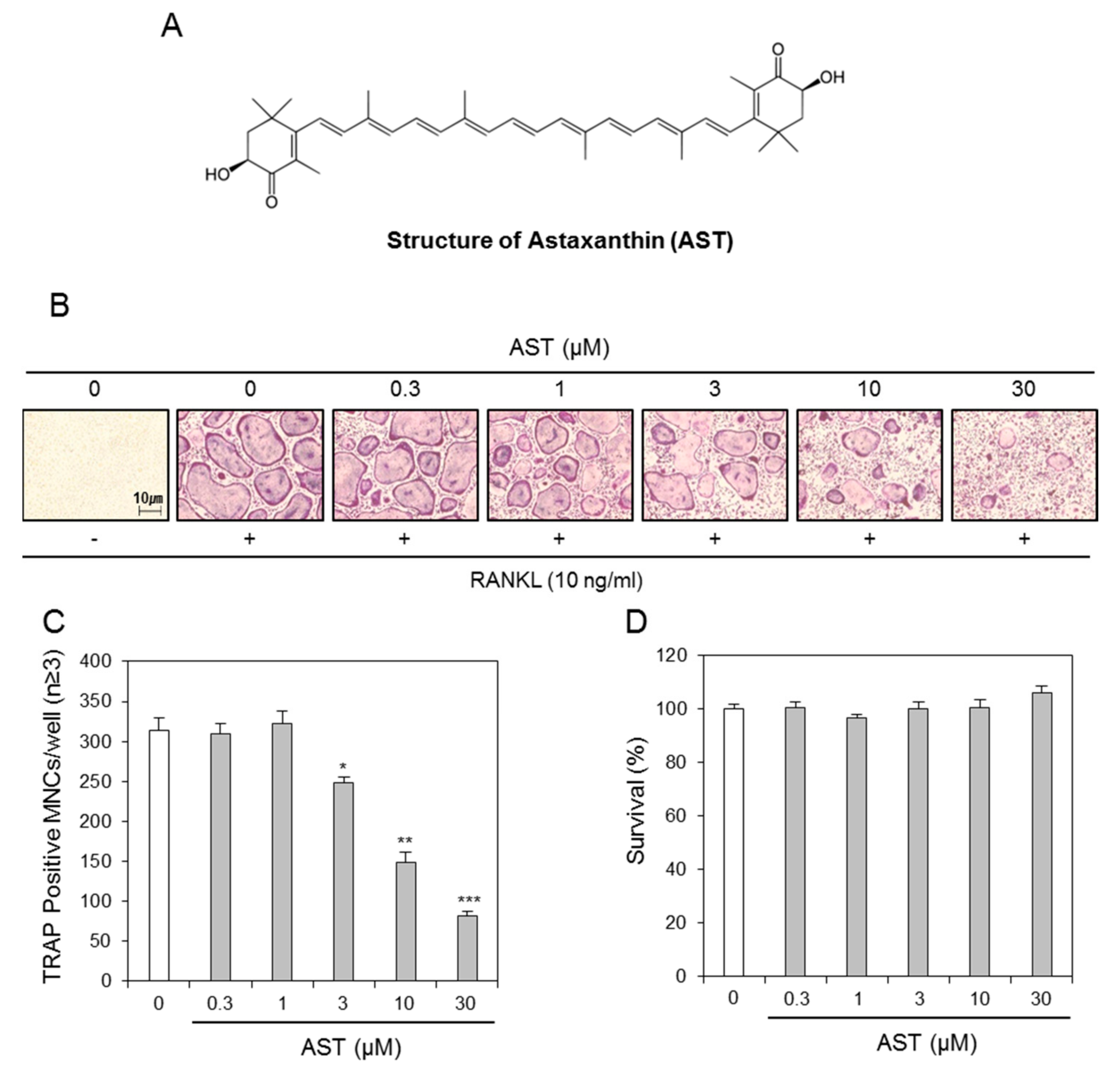
2.1. Effects of AST on Osteoclast Differentiation
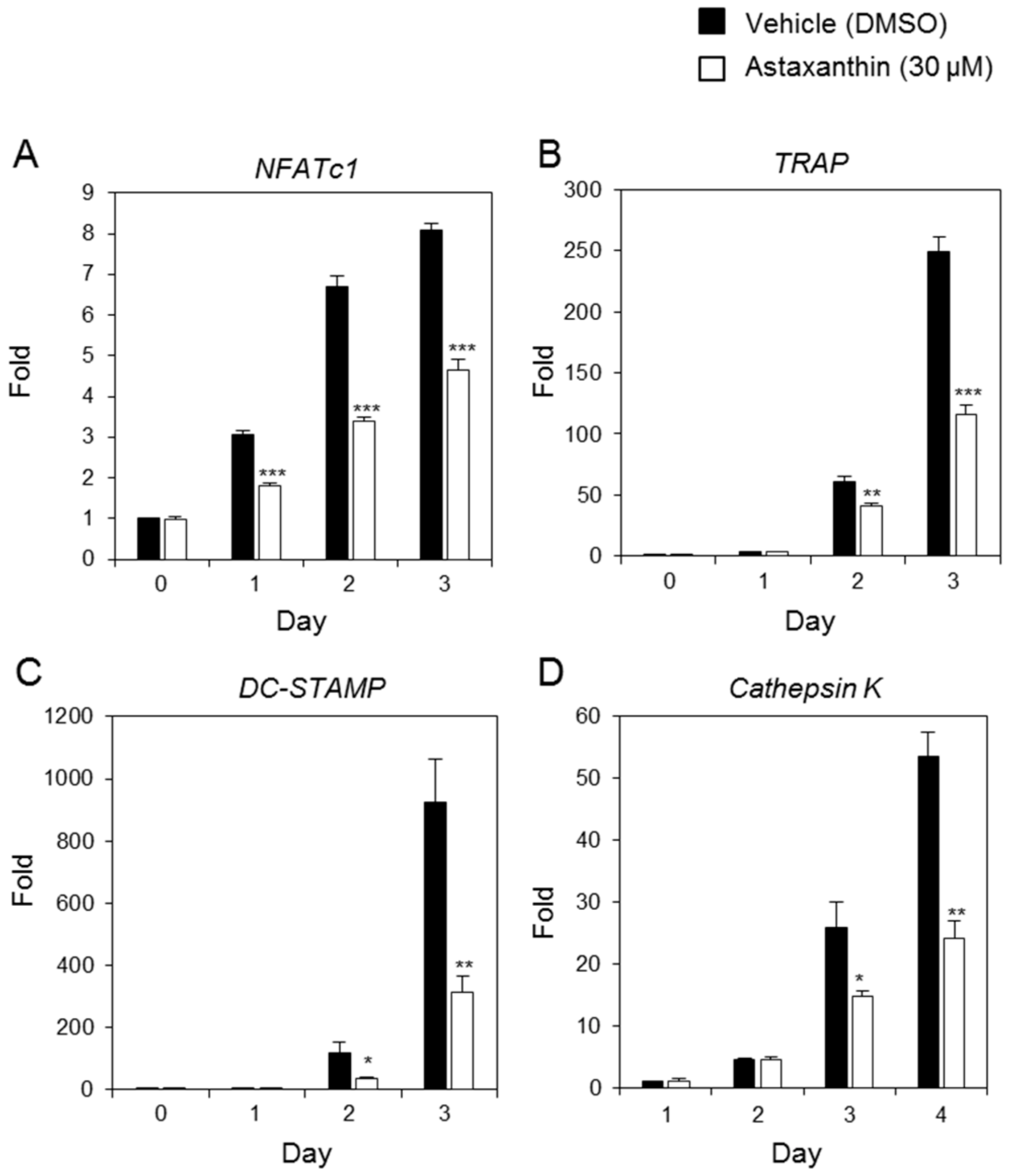
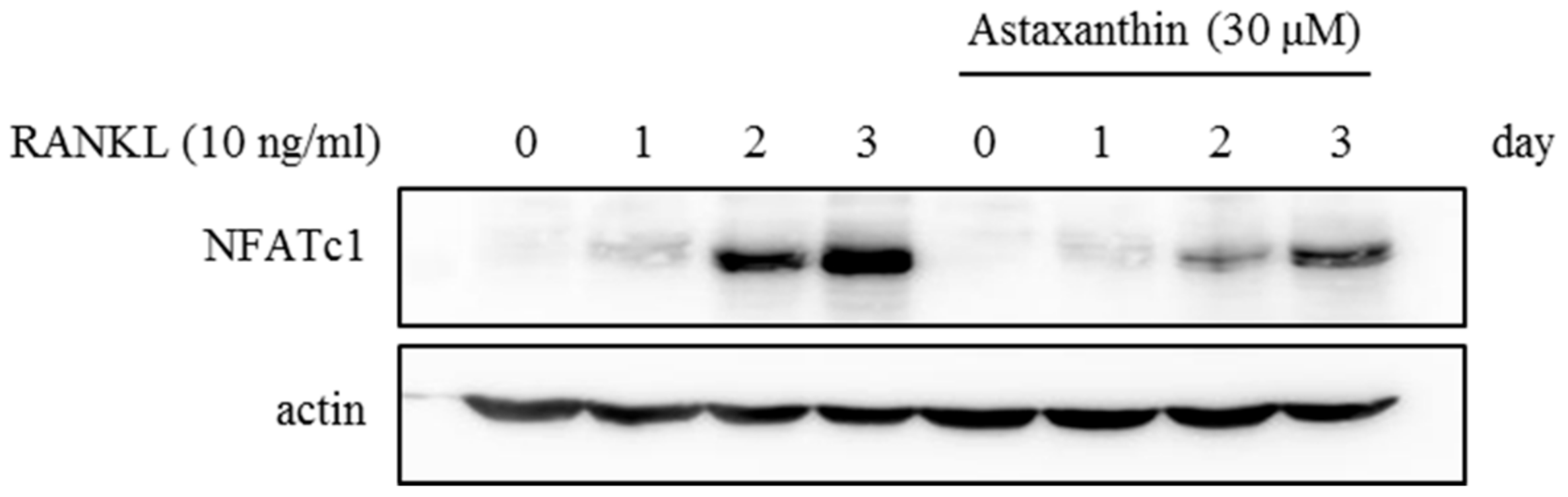
2.2. Effects of AST on RANKL-Induced mRNA Expression of Osteoclast-Specific Genes
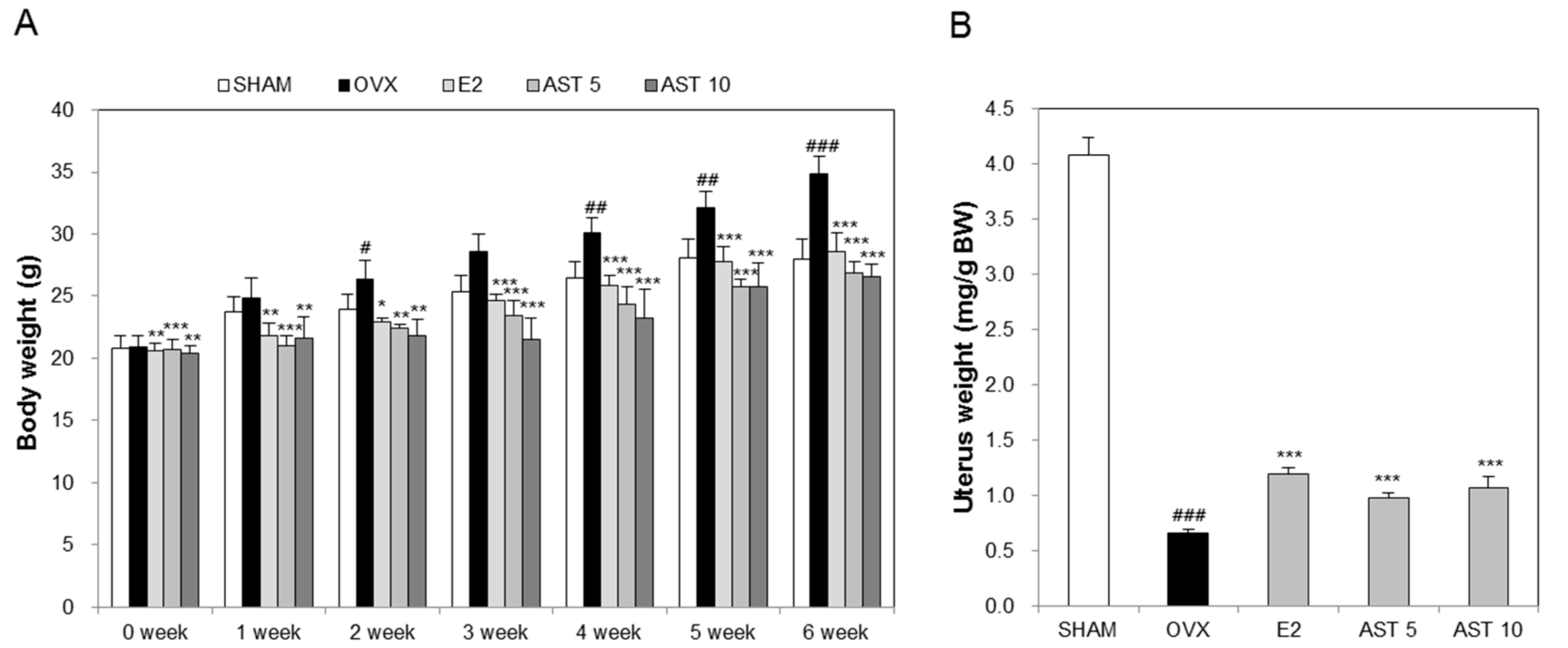
2.3. Effects of AST on Body Weight and Uterus Weight in Osteoporotic Mice
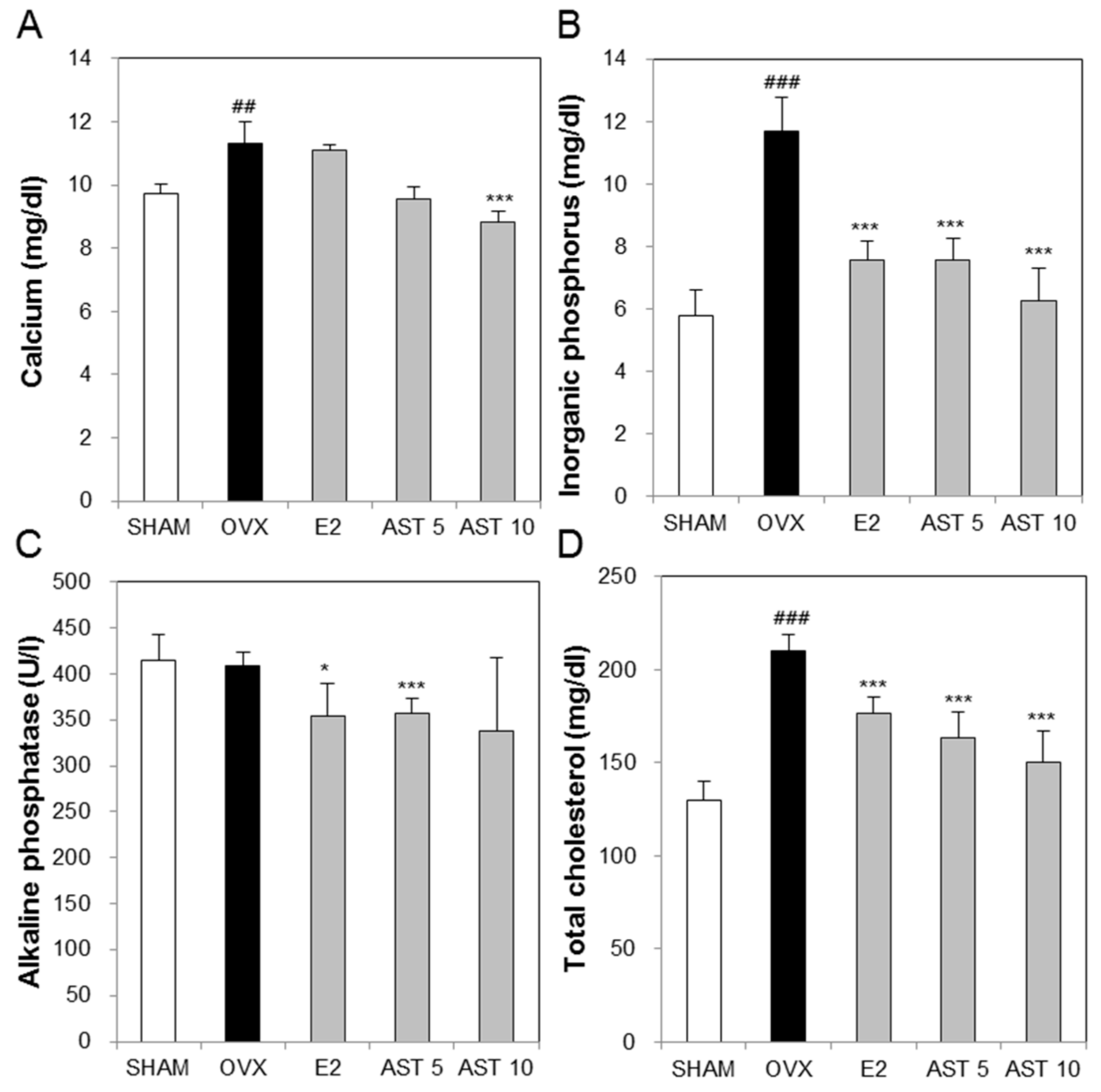
2.4. Effects of AST on Biochemical Markers in Osteoporotic Mice
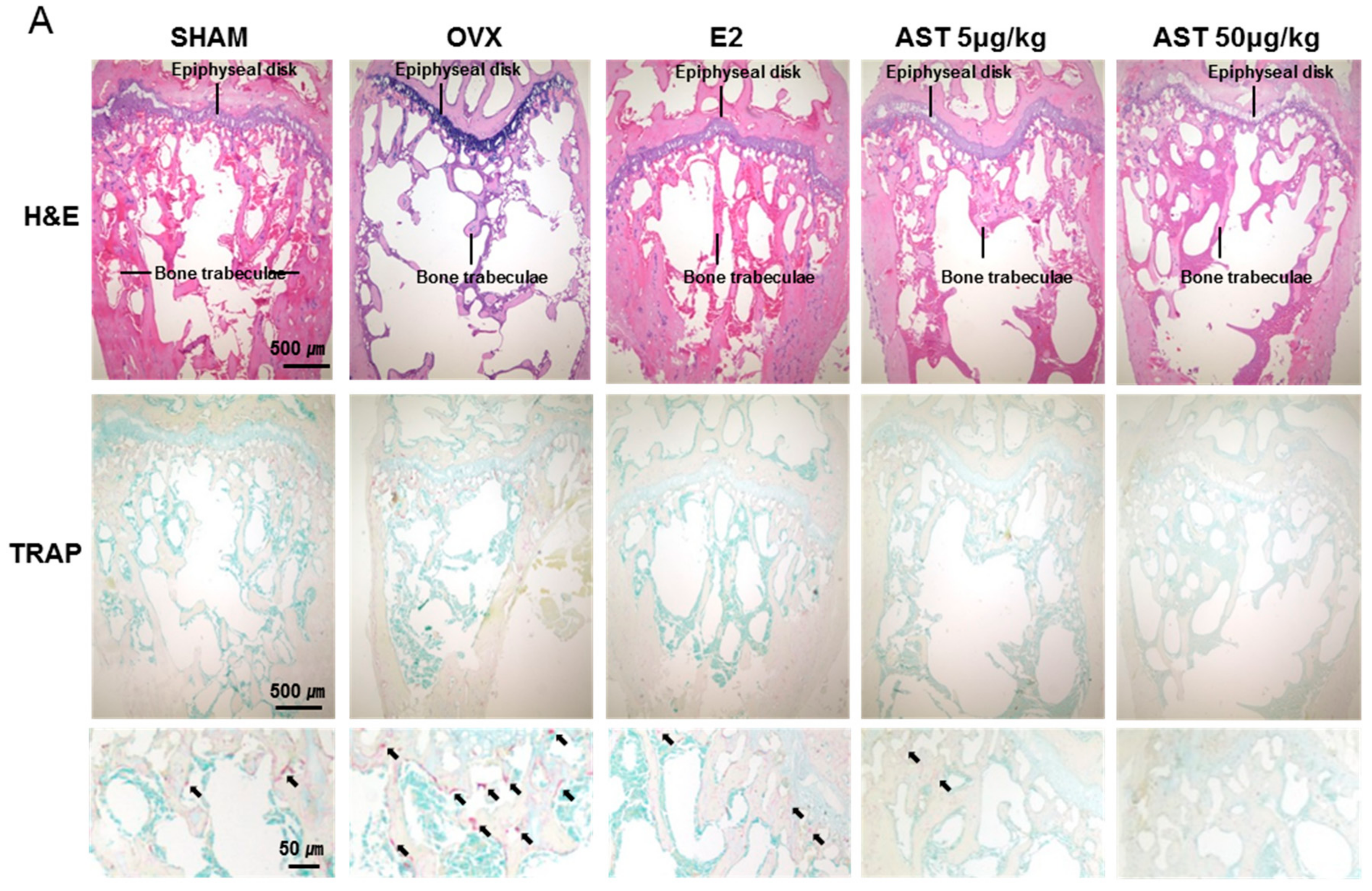
2.5. Effects of AST on the Level of TRAP in Osteoporotic Mice
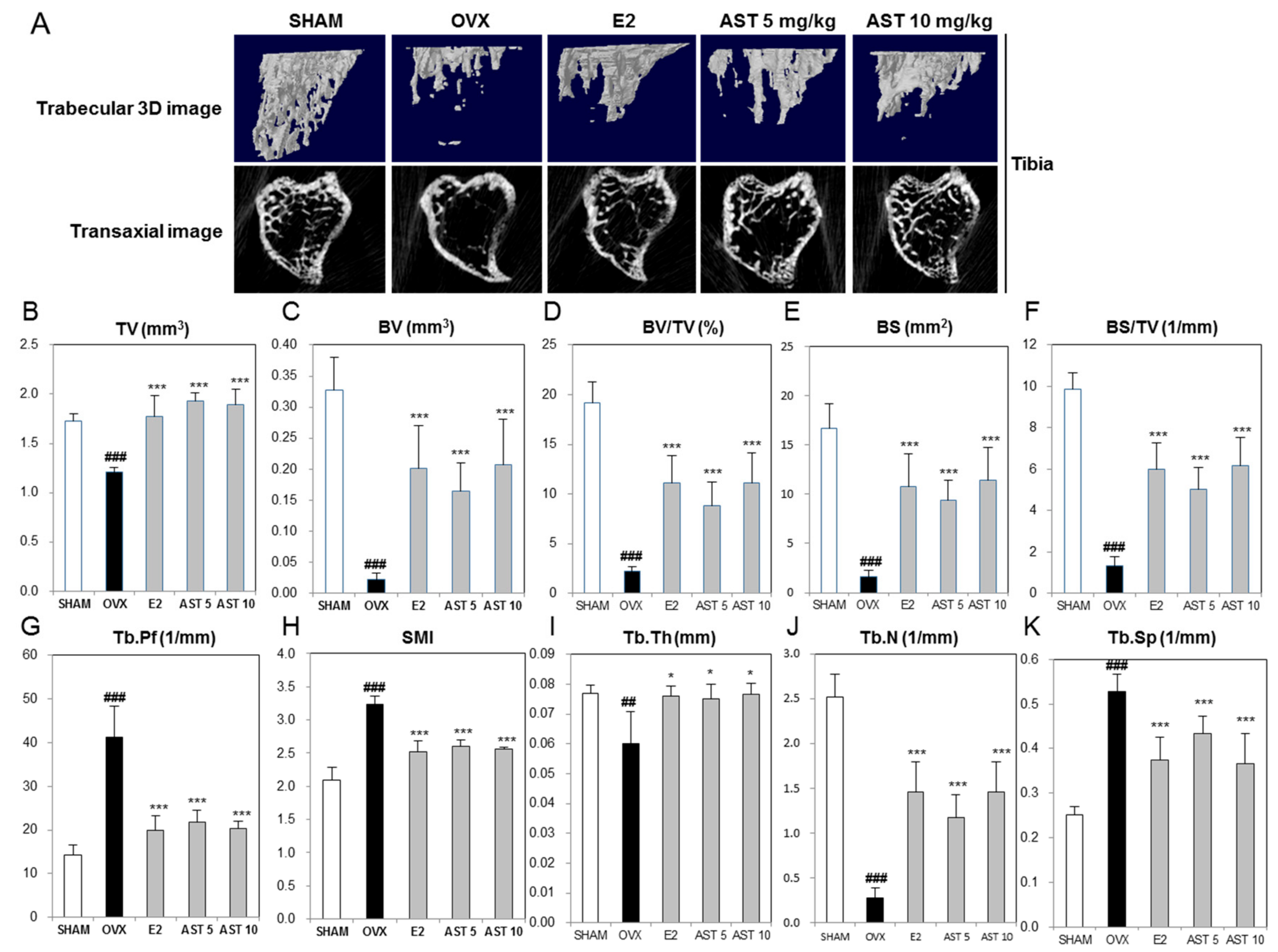
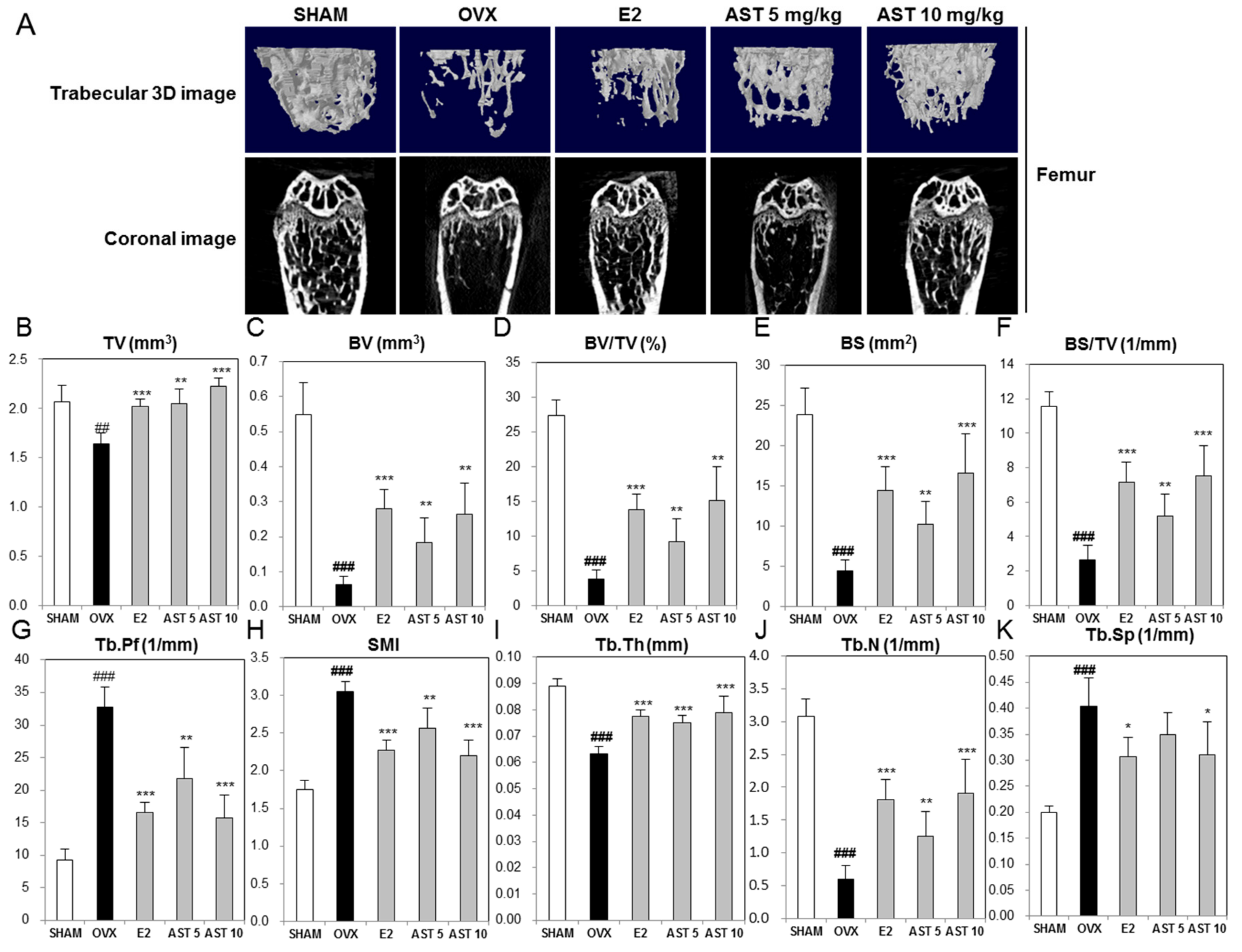
2.6. Effect of AST on the Micro-Architecture of Proximal Tibia and Digital Femur
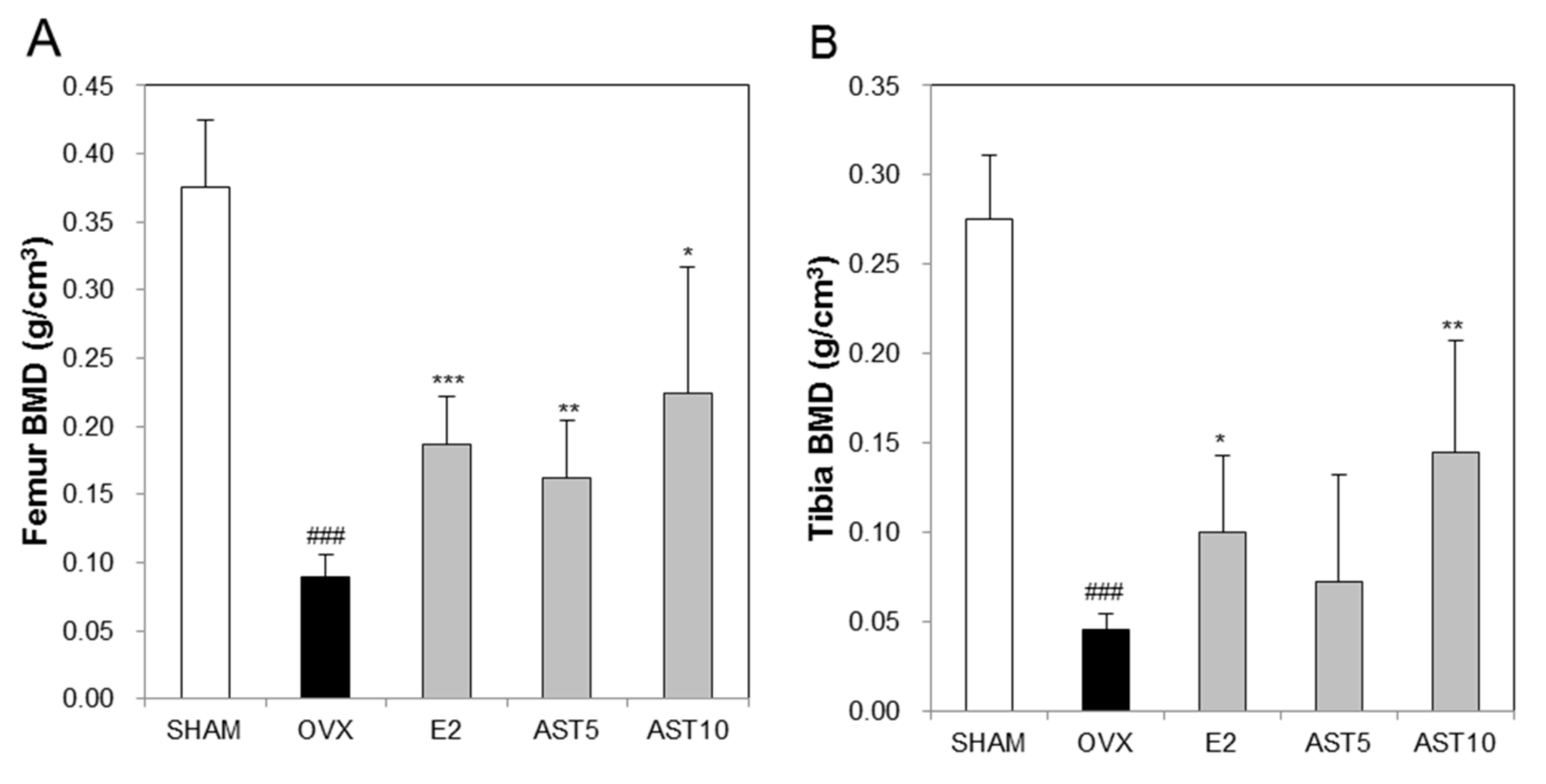
2.7. Effect of AST on Bone Mineral Density in Osteoporotic Mice
2.8. Effect of AST on Histological Changes in Osteoporotic Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Cultures and Osteoclast Differentiation
4.3. Cytotoxicity Assay for Astaxanthin
4.4. Tartrate-Resistant acid Phosphatase (TRAP) Staining Assay
4.5. Real-Time PCR
4.6. Western Blot Analysis
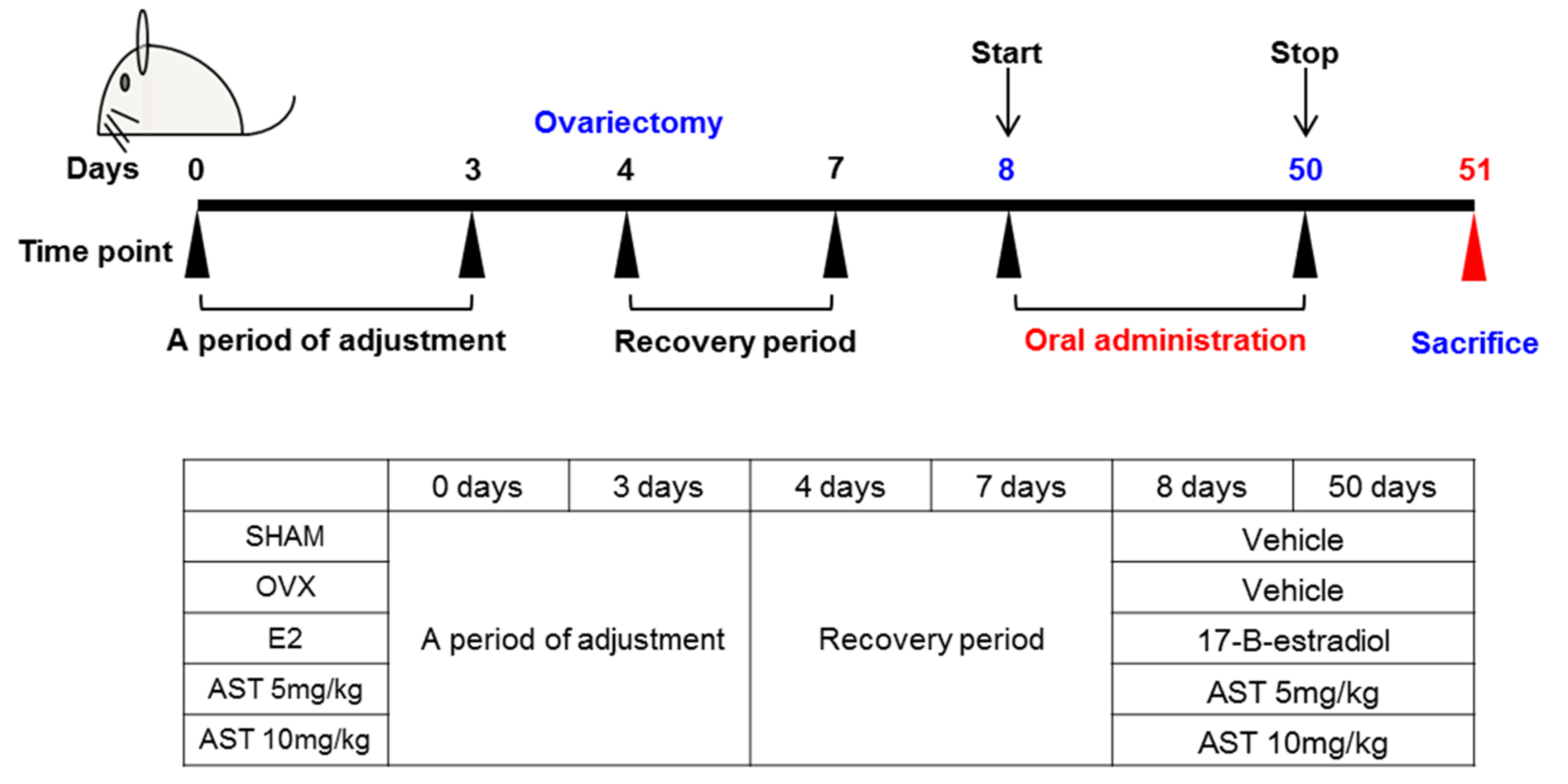
4.7. Experimental Animals
4.8. Measurements of Serum Ca, IP, ALP, and TCHO
4.9. Measurements of TRAP and BALP in Serum by ELISA
4.1.10. Analysis of Trabecular Bone Morphometric Parameters
4.1.11. Histological Analysis
4.1.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kanis, J.A.; WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Weycker. D.; Li, X.; Barron, R.; Bornheimer, R.; Chandler, D. Hospitalizations for osteoporosis-related fractures: Economic costs and clinical outcomes. Bone Rep. 2016, 5, 186–191. [Google Scholar]
- Fitzpatrick, L.A. Estrogen therapy for postmenopausal osteoporosis. Arq. Bras. Endocrinol. Metab. 2006, 50, 705–719. [Google Scholar] [CrossRef]
- Zhao, R. Immune regulation of osteoclast function in postmenopausal osteoporosis: A critical interdisciplinary perspective. Int. J. Med. Sci. 2012, 9, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.Y.P.; Flores, M.V.; Orozco, L.; Cruz, R.V. Molecular aspects of bone remodeling. Top. Osteoporos. 2013. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Takayanagi, H. The role of NFAT in osteoclast formation. Ann. N. Y. Acad. Sci. 2007, 1116, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Yeon, J.T.; Choi, S.W.; Moon, S.H.; Ryu, B.J.; Yu, R.; Park, S.J.; Kim, S.H.; Son, Y.J. Decursin inhibits osteoclastogenesis by downregulating NFATc1 and blocking fusion of pre-osteoclasts. Bone 2015, 81, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [PubMed]
- Lobo, R.A. Benefits and risks of estrogen replacement therapy. Am. J. Obstet. Gynecol. 1995, 173, 982–989. [Google Scholar] [CrossRef]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar]
- Yamaguchi, M.; Levy, R.M. Combination of alendronate and genistein synergistically suppresses osteoclastic differentiation of RAW267.4 cells in vitro. Exp. Ther. Med. 2017, 14, 1769–1774. [Google Scholar] [CrossRef] [PubMed]
- Ishimi, Y.; Miyaura, C.; Ohmura, M.; Onoe, Y.; Sato, T.; Uchiyama, Y.; Ito, M.; Wang, X.; Suda, T.; Ikegami, S. Selective effects of genistein, a soybean isoflavone, on B-lymphopoiesis and bone loss caused by estrogen deficiency. Endocrinology 1999, 140, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Atteritano, M.; Mazzaferro, S.; Bitto, A.; Cannata, M.L.; D’Anna, R.; Squadrito, F.; Macrì, I.; Frisina, A.; Frisina, N.; Bagnato, G. Genistein effects on quality of life and depression symptoms in osteopenic postmenopausal women: A 2-year randomized, double-blind, controlled study. Osteoporos. Int. 2014, 25, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Arcoraci, V.; Atteritano, M.; Squadrito, F.; D’Anna, R.; Marini, H.; Santoro, D.; Minutoli, L.; Messina, S.; Altavilla, D.; Bitto, A. Antiosteoporotic Activity of Genistein Aglycone in Postmenopausal Women: Evidence from a Post-Hoc Analysis of a Multicenter Randomized Controlled Trial. Nutrients 2017, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Kurashige, M.; Okimasu, E.; Inoue, M.; Utsumi, K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol. Chem. Phys. Med. NMR 1990, 22, 27–38. [Google Scholar] [PubMed]
- Hwang, Y.H.; Hong, S.G.; Mun, S.K.; Kim, S.J.; Lee, S.J.; Kim, J.J.; Kang, K.Y.; Yee, S.T. The Protective Effects of Astaxanthin on the OVA-Induced Asthma Mice Model. Molecules 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Takahara, T.; Fujino, M.; Fukuhara, Y.; Sugiyama, T.; Li, X.K.; Takahara, S. Astaxanthin prevents ischemia-reperfusion injury of the steatotic liver in mice. PLoS ONE 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, S.; Bi, J.; Gu, J.; Deng, Y.; Liu, C. Astaxanthin pretreatment attenuates acetaminophen-induced liver injury in mice. Int. Immunopharmacol. 2017, 45, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, J.; Liu, T.; Jiao, G.; Li, C.; Hu, B. Astaxanthin inhibits proliferation and promotes apoptosis of A549 lung cancer cells via blocking JAK1/STAT3 pathway. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2016, 32, 784–788. [Google Scholar] [PubMed]
- Wen, X.; Xiao, L.; Zhong, Z.; Wang, L.; Li, Z.; Pan, X.; Liu, Z. Astaxanthin acts via LRP-1 to inhibit inflammation and reverse lipopolysaccharide-induced M1/M2 polarization of microglial cells. Oncotarget 2017, 8, 69370–69385. [Google Scholar] [CrossRef] [PubMed]
- Balci Yuce, H.; Lektemur Alpan, A.; Gevrek, F.; Toker, H. Investigation of the effect of astaxanthin on alveolar bone loss in experimental periodontitis. J. Periodontal Res. 2018, 53, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Blume, S.W.; Curtis, J.R. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos. Int. 2011, 22, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, S.H.; Ha Kim, J.; Choi, Y.; Kim, N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol. Endocrinol. 2008, 22, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.W.; Yutzey, K.E. NFATc1 expression in the developing heart valves is responsive to the RANKL pathway and is required for endocardial expression of cathepsin K. Dev. Biol. 2006, 292, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Lelovas, P.P.; Xanthos, T.T.; Thoma, S.E.; Lyritis, G.P.; Dontas, I.A. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008, 58, 424–430. [Google Scholar] [PubMed]
- Xu, Y.; Chen, T.; Li, X.; Qu, Y.K.; An, J.N.; Zheng, H.X.; Zhang, Z.J.; Lin, N. Salvia miltiorrhiza bunge increases estrogen level without side effects on reproductive tissues in immature/ovariectomized mice. Aging 2016, 9, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, Y.; Guo, B.; Tsang, M.C.; Tu, F.; Dai, Y.; Yao, Z.; Zheng, L.; Xie, X.; Wang, N.; et al. In Vivo Screening for Anti-Osteoporotic Fraction from Extract of Herbal Formula Xianlinggubao in Ovariectomized Mice. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Kanaya, N.; Kubo, M.; Liu, Z.; Chu, P.; Wang, C.; Yuan, Y.C.; Chen, S. Protective Effects of White Button Mushroom (Agaricus bisporus) against Hepatic Steatosis in Ovariectomized Mice as a Model of Postmenopausal Women. PLoS ONE 2011. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Kang, K.Y.; Kim, J.J.; Lee, S.J.; Son, Y.J.; Paik, S.H.; Yee, S.T. Effects of Hot Water Extracts from Polygonum multiflorum on Ovariectomy Induced Osteopenia in Mice. Evid Based Complement. Altern. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, E.Y.; Lee, B.; Min, J.H.; Song, D.U.; Lim, J.M.; Eom, J.W.; Yeom, M.; Jung, H.S.; Sohn, Y. The effects of Lycii Radicis Cortex on RANKL-induced osteoclast differentiation and activation in RAW 264.7 cells. Int. J. Mol. Med. 2016, 37, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.C.; van Bezooijen, R.L.; Karperien, M.; Papapoulos, S.E.; Löwik, C.W. Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J. Bone Miner. Res. 2002, 17, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, E.; Liedert, A.; Heilmann, A.; Wehner, T.; Bindl, R.; Fischer, L.; Haffner-Luntzer, M.; Jakob, F.; Schinke, T.; Amling, M.; et al. The impact of low-magnitude high-frequency vibration on fracture healing is profoundly influenced by the oestrogen status in mice. Dis. Model. Mech. 2015, 8, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Cho, L.; Shin, J.S.; Lee, J.; Ha, I.H.; Park, H.J.; Lee, S.K. Effects of JSOG-6 on protection against bone loss in ovariectomized mice through regulation of osteoblast differentiation and osteoclast formation. BMC Complement. Altern. Med. 2014, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qiu, X.M.; Gui, Y.Y.; Xu, Y.P.; Gober, H.J.; Li, D.J. Bu-Shen-Ning-Xin Decoction ameliorated the osteoporotic phenotype of ovariectomized mice without affecting the serum estrogen concentration or uterus. Drug. Des. Develop. Ther. 2015, 9, 5019–5031. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, W. Effects of tanshinol on markers of bone turnover in ovariectomized rats and osteoblast cultures. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Nordin, B.E. Calcium and osteoporosis. Nutrition 1997, 13, 664–686. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Riggs, B.L.; DeLuca, H.F. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 1980, 51, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Penido, M.G.M.G.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.L.; Qin, R.Z.; Yang, Y.X.; Huang, K.B.; Yang, X.W. Polydatin possesses notable anti‑osteoporotic activity via regulation of OPG, RANKL and β-catenin. Mol. Med. Rep. 2016, 14, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cao, L.; Xia, L.; Wu, Q.; Wang, J.; Wang, X.; Xu, L.; Zhou, Y.; Xu, Y.; Jiang, X. Evaluation of Osteogenesis and Angiogenesis of Icariin in Local Controlled Release and Systemic Delivery for Calvarial Defect in Ovariectomized Rats. Sci. Rep. 2017, 7, 5077. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Casimirri, F.; Pascal, G.; Tortelli, O.; Morselli Labate, A.; Bertazzo, D.; Vicennati, V.; Gaddi, A.; Virgilio Menopause Health Group. Influence of menopause on blood cholesterol levels in women: The role of body composition, fat distribution and hormonal milieu. J. Int. Med. 1997, 241, 195–203. [Google Scholar] [CrossRef]
- Trimpou, P.; Odén, A.; Simonsson, T.; Wilhelmsen, L.; Landin-Wilhelmsen, K. High serum total cholesterol is a long-term cause of osteoporotic fracture. Osteoporos. Int. 2011, 22, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Bachmann, K.A. An investigation of the relationship between estrogen, estrogen metabolites and blood cholesterol levels in ovariectomized rats. J. Pharmacol. Exp. Ther. 1998, 286, 561–568. [Google Scholar] [PubMed]
- Nuttall, M.E.; Bradbeer, J.N.; Stroup, G.B.; Nadeau, D.P.; Hoffman, S.J.; Zhao, H.; Rehm, S.; Gowen, M. Idoxifene: A novel selective estrogen receptor modulator prevents bone loss and lowers cholesterol levels in ovariectomized rats and decreases uterine weight in intact rats. Endocrinology 1998, 139, 5224–5234. [Google Scholar] [CrossRef] [PubMed]
- D Lim, D.W.; Kim, J.G.; Kim, Y.T. Effects of dietary isoflavones from Puerariae radix on lipid and bone metabolism in ovariectomized rats. Nutrients 2013, 5, 2734–2746. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L. Microarchitecture, the key to bone quality. Rheumatology 2009. [Google Scholar] [CrossRef] [PubMed]
- Kulak, C.A.; Dempster, D.W. Bone histomorphometry: A concise review for endocrinologists and clinicians. Arq. Bras. Endocrinol. Metabol. 2010, 54, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Vidal, B.; Pinto, A.; Galvão, M.J.; Santos, A.R.; Rodrigues, A.; Cascão, R.; Abdulghani, S.; Caetano-Lopes, J.; Ferreira, A.; Fonseca, J.E.; Canhao, H. Bone histomorphometry revisited. Acta Reumatol. Port. 2012, 37, 294–300. [Google Scholar] [PubMed]
- Chappard, C.; Marchadier, A.; Benhamou, L. Interindividual and intraspecimen variability of 3-D bone microarchitectural parameters in iliac crest biopsies imaged by conventional micro-computed tomography. J. Bone Miner. Metab. 2008, 26, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Xiang, A.; Kanematsu, M.; Kumar, S.; Yamashita, D.; Kaise, T.; Kikkawa, H.; Asano, S.; Kinoshita, M. Changes in micro-CT 3D bone parameters reflect effects of a potent cathepsin K inhibitor (SB-553484) on bone resorption and cortical bone formation in ovariectomized mice. Bone 2007, 40, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Spilmont, M.; Léotoing, L.; Davicco, M.J.; Lebecque, P.; Mercier, S.; Miot-Noirault, E.; Pilet, P.; Rios, L.; Wittrant, Y.; Coxam, V. Pomegranate seed oil prevents bone loss in a mice model of osteoporosis, through osteoblastic stimulation, osteoclastic inhibition and decreased inflammatory status. J. Nutr. Biochem. 2013, 24, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.J. Overview of role of BMD measurements in managing osteoporosis. Semin. Nucl. Med. 1997, 27, 197–209. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [PubMed]


| Gene of Interest | Primer Sequence (5′→3′) | |
|---|---|---|
| Sense | Anti-Sense | |
| NFATc1 | GGGTCAGTGTGACCGAAGAT | GGAAGTCAGAAGTGGGTGGA |
| cathepsin K | GGCCAACTCAAGAAGAAAAC | GTGCTTGCTTCCCTTCTGG |
| DC-STAMP | CCAAGGAGTCGTCCATGATT | GGCTGCTTTGATCGTTTCTC |
| TRAP | GATGACTTTGCCAGTCAGCA | ACATAGCCCACACCGTTCTC |
| GAPDH | AACTTTGGCATTGTGGAAGG | ACACATTGGGGGTAGGAACA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, Y.-H.; Kim, K.-J.; Kim, S.-J.; Mun, S.-K.; Hong, S.-G.; Son, Y.-J.; Yee, S.-T. Suppression Effect of Astaxanthin on Osteoclast Formation In Vitro and Bone Loss In Vivo. Int. J. Mol. Sci. 2018, 19, 912. https://doi.org/10.3390/ijms19030912
Hwang Y-H, Kim K-J, Kim S-J, Mun S-K, Hong S-G, Son Y-J, Yee S-T. Suppression Effect of Astaxanthin on Osteoclast Formation In Vitro and Bone Loss In Vivo. International Journal of Molecular Sciences. 2018; 19(3):912. https://doi.org/10.3390/ijms19030912
Chicago/Turabian StyleHwang, Yun-Ho, Kwang-Jin Kim, Su-Jin Kim, Seul-Ki Mun, Seong-Gyeol Hong, Young-Jin Son, and Sung-Tae Yee. 2018. "Suppression Effect of Astaxanthin on Osteoclast Formation In Vitro and Bone Loss In Vivo" International Journal of Molecular Sciences 19, no. 3: 912. https://doi.org/10.3390/ijms19030912