Resveratrol Suppresses the Growth and Enhances Retinoic Acid Sensitivity of Anaplastic Thyroid Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. RA Resistance of Anaplastic Thyroid Cancer (ATC) Cell Lines
2.2. Resveratrol Suppresess the Growth of THJ-16T and THJ-21T Cells
2.3. Resveratrol Resistance of THJ-11T Cells
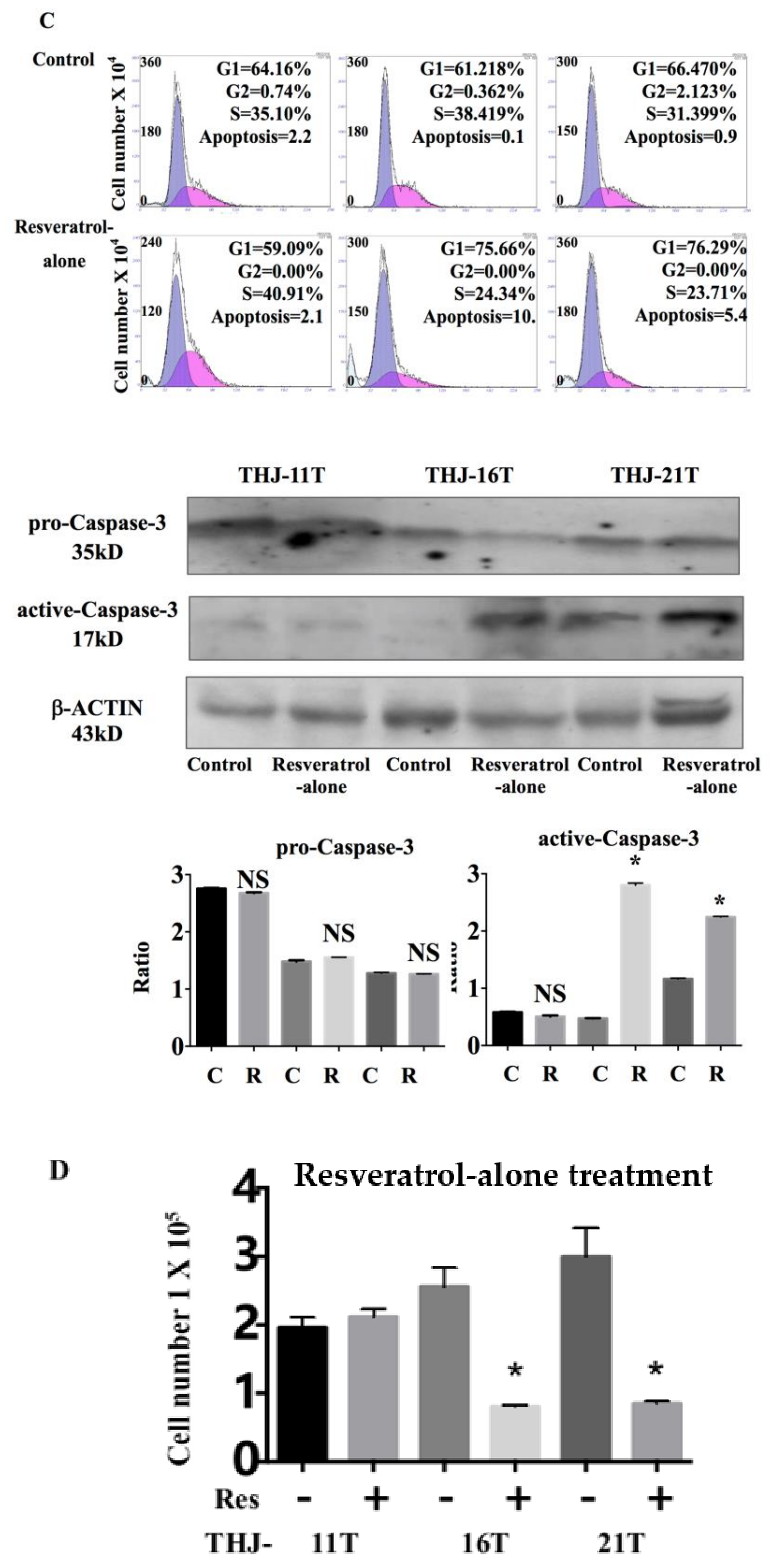
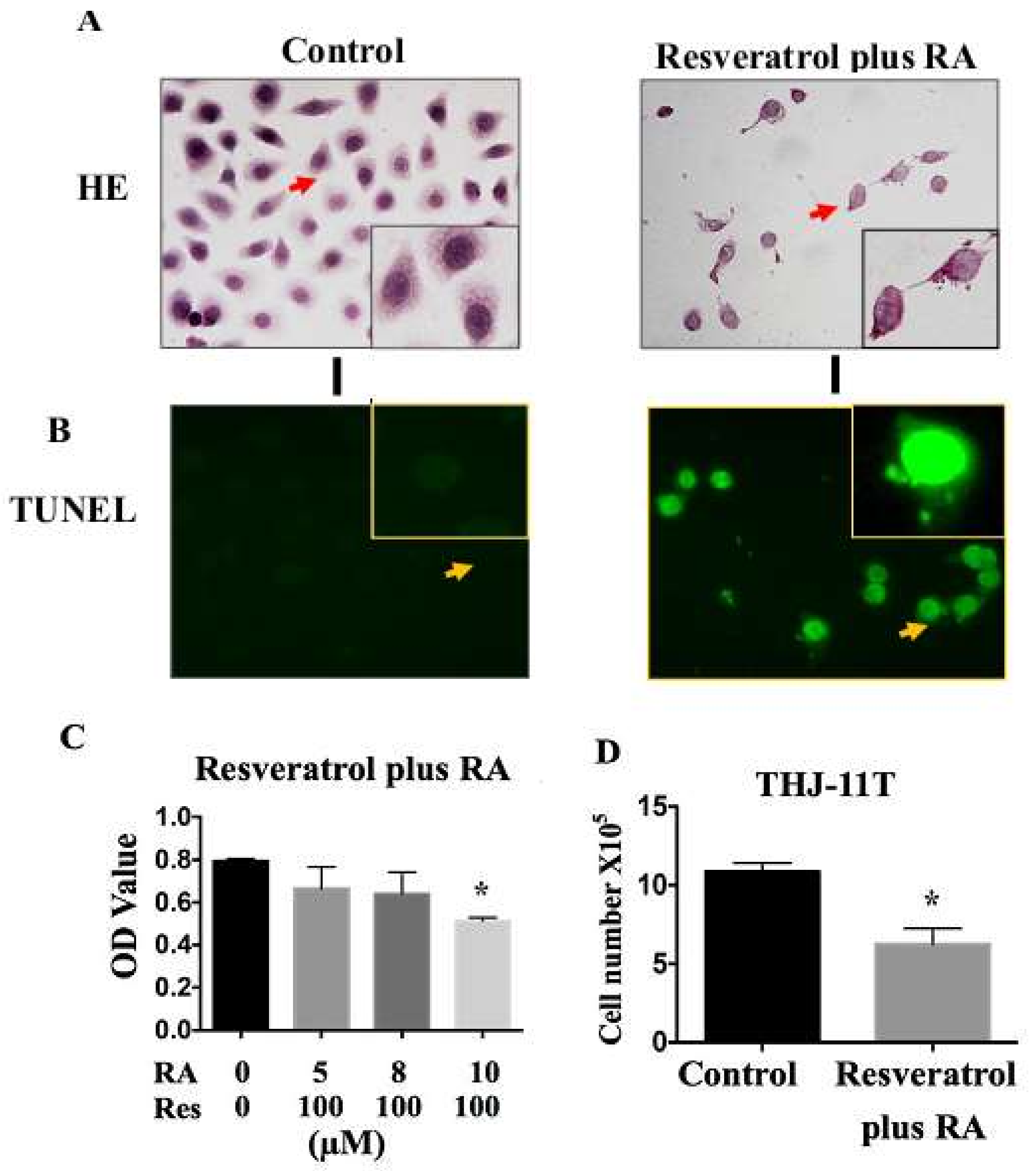
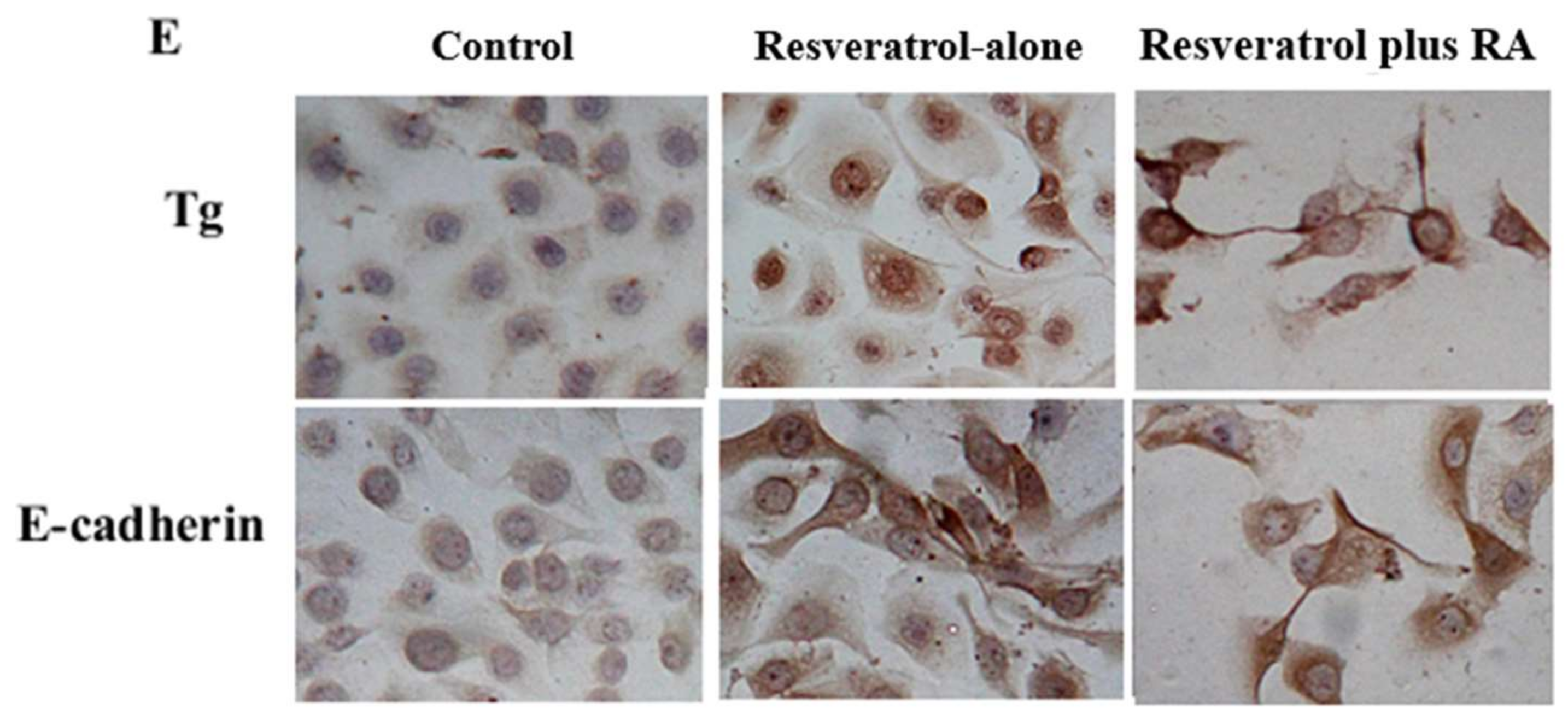
2.4. Resveratrol Reverses Retinoic Acid Resistance of THJ-11T Cells
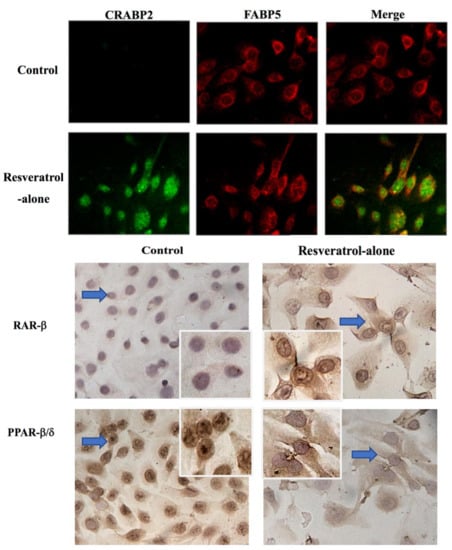
2.5. Significant Upregulation of Cellular Retinoic Acid-Binding Protein 2 (CRABP2) in Resveratrol-Treated THJ-11T Cells
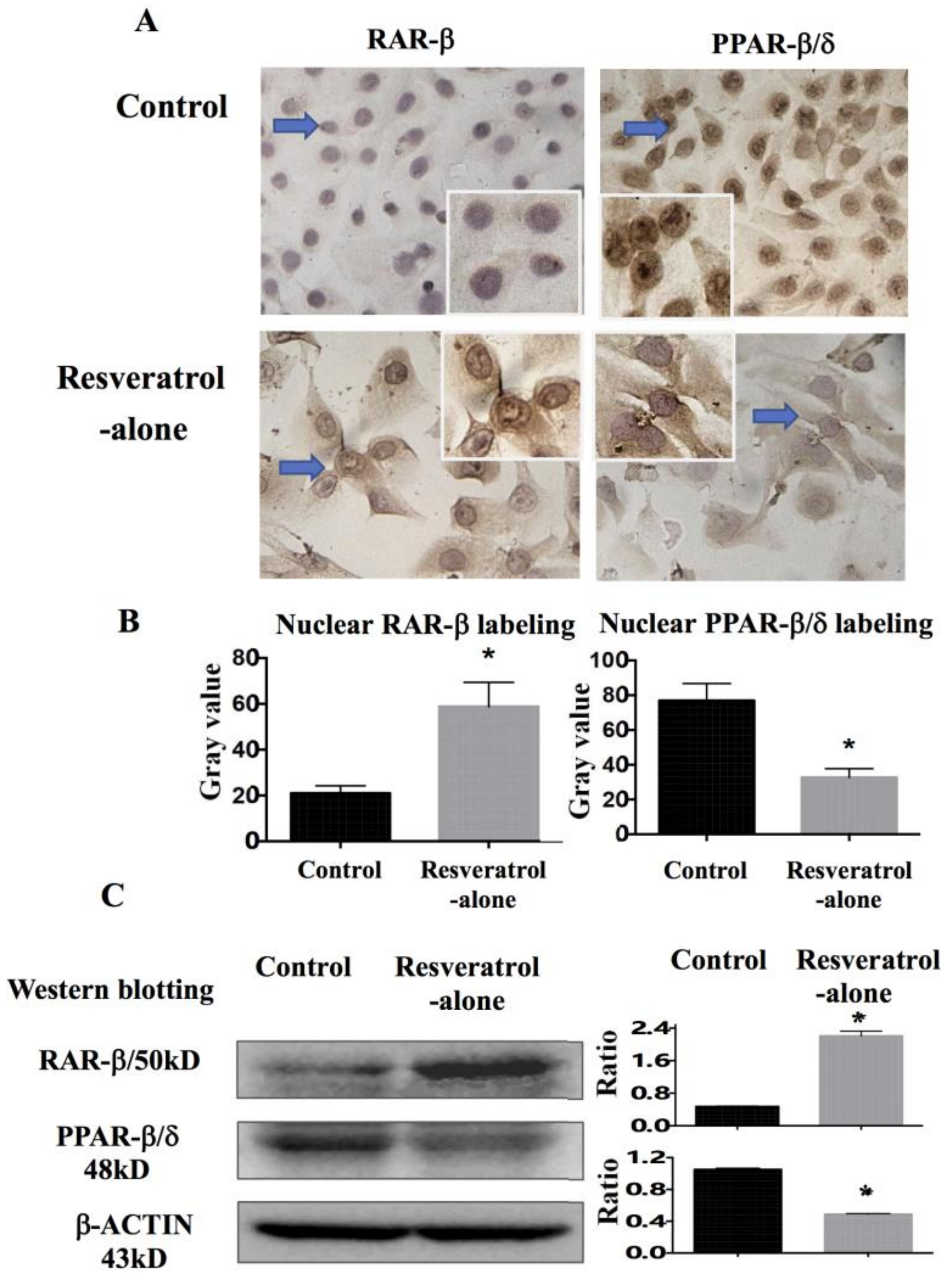
2.6. Differential Response of Retinoic Acid Receptor (RAR)-β and Peroxisome Proliferator-Activated Receptor (PPAR)-β/δ to Resveratrol
3. Discussion
4. Materials and Methods
4.1. Thyroid Cancer Cell Lines and Culture
4.2. Reagents and Cell Treatments
4.3. Evaluation of Cell Proliferation and Death
4.4. Protein Preparation and Western Blotting
4.5. Immunocytochemical Staining and Double Immunofluorescent Labeling
4.6. RNA Isolation and RT-PCR
4.7. ImageJ-based Quantification of Nuclear Translocation
4.8. RA Sensitivity Assay of Resveratrol-Treated THJ-11T Cells
4.9. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- La Vecchia, C.; Malvezzi, M.; Bosetti, C.; Garavello, W.; Bertuccio, P.; Levi, F.; Negri, E. Thyroid cancer mortality and incidence: A global overview. Int. J. Cancer 2015, 136, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Zeng, H.; Zhang, S.; Chen, W. Estimates of cancer incidence and mortality in China, 2013. Chin. J. Cancer 2017, 36, 66. [Google Scholar] [CrossRef] [PubMed]
- Hedinger, C.; Williams, E.D.; Sobin, L.H. The WHO histological classification of thyroid tumors: A commentary on the second edition. Cancer 1989, 63, 908–911. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Molinaro, E.; Romei, C.; Biagini, A.; Sabini, E.; Agate, L.; Mazzeo, S.; Materazzi, G.; Sellari-Franceschini, S.; Ribechini, A.; Torregrossa, L.; et al. Anaplastic thyroid carcinoma: From clinicopathology to genetics and advanced therapies. Nat. Rev. Endocrinol. 2017, 13, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.L.; Gandhi, A.; Scott-Coombes, D.; Perros, P. Management of thyroid cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S150–S160. [Google Scholar] [CrossRef] [PubMed]
- Bisof, V.; Rakusic, Z.; Despot, M. Treatment of patients with anaplastic thyroid cancer during the last 20 years: Whether any progress has been made? Eur. Arch. Otorhinolaryngol. 2015, 272, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Schmutzler, C.; Kohrle, J. Retinoic acid redifferentiation therapy for thyroid cancer. Thyroid 2000, 10, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, M.C.; Cosco, D.; Celia, C.; Tudose, A.; Mare, R.; Paolino, D.; Fresta, M. Anticancer activity of all-trans retinoic acid-loaded liposomes on human thyroid carcinoma cells. Colloids Surf. B Biointerfaces 2017, 150, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Courbon, F.; Zerdoud, S.; Bastie, D.; Archambaud, F.; Hoff, M.; Eche, N.; Berry, I.; Caron, P. Defective efficacy of retinoic acid treatment in patients with metastatic thyroid carcinoma. Thyroid 2006, 16, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Malehmir, M.; Haghpanah, V.; Larijani, B.; Ahmadian, S.; Alimoghaddam, K.; Heshmat, R.; Ghavamzadeh, A.; Adabi, K.; Ghaffari, S.H. Multifaceted suppression of aggressive behavior of thyroid carcinoma by all-trans retinoic acid induced re-differentiation. Mol. Cell. Endocrinol. 2012, 348, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, H.; Wang, X.W.; Wu, D.C.; Chen, X.Y.; Liu, J. Resveratrol promotes differentiation and induces Fas-independent apoptosis of human medulloblastoma cells. Neurosci. Lett. 2003, 351, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar] [PubMed]
- Zhang, P.; Li, H.; Yang, B.; Yang, F.; Zhang, L.L.; Kong, Q.Y.; Chen, X.Y.; Wu, M.L.; Liu, J. Biological significance and therapeutic implication of resveratrol-inhibited Wnt, Notch and STAT3 signaling in cervical cancer cells. Genes Cancer 2014, 5, 154–164. [Google Scholar] [PubMed]
- Zhong, L.X.; Zhang, Y.; Wu, M.L.; Liu, Y.N.; Zhang, P.; Chen, X.Y.; Kong, Q.Y.; Liu, J.; Li, H. Resveratrol and STAT inhibitor enhance autophagy in ovarian cancer cells. Cell Death Discov. 2016, 2, 15071. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Li, H.; Liu, J.; Wu, M.L.; Chen, X.Y.; Liu, L.H.; Wang, Q. Inactivated Wnt signaling in resveratrol-treated epidermal squamous cancer cells and its biological implication. Oncol. Lett. 2017, 14, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T.; Berry, D.C.; Shaw, N.S.; Travis, S.N.; Noy, N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 2007, 129, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Lorenzo, G.D.; Scarpati, G.D.; Buonerba, C. Anaplastic thyroid carcinoma: A comprehensive review of current and future therapeutic options. World J. Clin. Oncol. 2011, 2, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Kebebew, E.; Greenspan, F.S.; Clark, O.H.; Woeber, K.A.; McMillan, A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer 2005, 103, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.M.; Jaskula-Sztul, R.; Ahmed, K.; Harrison, A.D.; Kunnimalaiyaan, M.; Chen, H. Resveratrol induces differentiation markers expression in anaplastic thyroid carcinoma via activation of Notch1 signaling and suppresses cell growth. Mol. Cancer Ther. 2013, 12, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, D.; Zhao, Q.C.; Shi, T.; Chen, J. Resveratrol inhibited non-small cell lung cancer through inhibiting STAT-3 signaling. Am. J. Med. Sci. 2016, 352, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, G.; Kaya-Dagistanli, F.; Ayla, S.; Demirci, S.; Eser, M.; Unal, Z.S.; Cengiz, M.; Oktar, H. Resveratrol can prevent CCl4-induced liver injury by inhibiting Notch signaling pathway. Histol. Histopathol. 2016, 31, 769–784. [Google Scholar] [PubMed]
- Yang, C.M.; Chen, Y.W.; Chi, P.L.; Lin, C.C.; Hsiao, L.D. Resveratrol inhibits BK-induced COX-2 transcription by suppressing acetylation of AP-1 and NF-κB in human rheumatoid arthritis synovial fibroblasts. Biochem. Pharmacol. 2017, 132, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Jiao, Y.; Guo, A.; Xu, X.; Qu, X.; Wang, S.; Zhao, J.; Li, Y.; Cao, Y. Resveratrol sensitizes glioblastoma-initiating cells to temozolomide by inducing cell apoptosis and promoting differentiation. Oncol. Rep. 2016, 35, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, W.; Wang, R.; Nan, Y.; Wang, Q.; Liu, W.; Jin, F. Resveratrol enhanced anticancer effects of cisplatin on non-small cell lung cancer cell lines by inducing mitochondrial dysfunction and cell apoptosis. Int. J. Oncol. 2015, 47, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Zhang, S.; Xu, H. Effect of slug-mediated down-regulation of E-cadherin on invasiveness and metastasis of anaplastic thyroid cancer cells. Med. Sci. Monit. 2017, 23, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, K.; Ananiev, J.; Aleksandrova, E.; Ignatova, M.M.; Gulubova, M. Expression of E-cadherin/β-catenin in epithelial carcinomas of the thyroid gland. Open Access Maced. J. Med. Sci. 2017, 5, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Fourati, A.; El Amine, O.; Ben Ayoub, W.; Cherni, I.; Goucha, A.; El May, M.V.; Gamoudi, A.; El May, A. Expression profile of biomarkers altered in papillary and anaplastic thyroid carcinoma: Contribution of Tunisian patients. Bull. Cancer 2017, 104, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.G.; Fortunati, N.; Pugliese, M.; Marano, F.; Ortoleva, L.; Poli, R.; Asioli, S.; Bandino, A.; Palestini, N.; Grange, C.; et al. Histone deacetylase inhibition modulates E-cadherin expression and suppresses migration and invasion of anaplastic thyroid cancer cells. J. Clin. Endocrinol. Metab. 2012, 97, E1150–E1159. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, R.; Xiao, W.; Sun, F.; Yuan, H.; Pan, Q. Cellular retinoic acid binding protein 2 is strikingly downregulated in human esophageal squamous cell carcinoma and functions as a tumor suppressor. PLoS ONE 2016, 11, e0148381. [Google Scholar] [CrossRef] [PubMed]
- Vreeland, A.C.; Levi, L.; Zhang, W.; Berry, D.C.; Noy, N. Cellular retinoic acid-binding protein 2 inhibits tumor growth by two distinct mechanisms. J. Biol. Chem. 2014, 289, 34065–34073. [Google Scholar] [CrossRef] [PubMed]
- Kannan-Thulasiraman, P.; Seachrist, D.D.; Mahabeleshwar, G.H.; Jain, M.K.; Noy, N. Fatty acid-binding protein 5 and PPARβ/δ are critical mediators of epidermal growth factor receptor-induced carcinoma cell growth. J. Biol. Chem. 2010, 285, 19106–19115. [Google Scholar] [CrossRef] [PubMed]
- Noy, N. Between death and survival: Retinoic acid in regulation of apoptosis. Annu. Rev. Nutr. 2010, 30, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Noy, N. Is PPARβ/δ a Retinoid Receptor? PPAR Res. 2007, 2007, 73256. [Google Scholar] [CrossRef] [PubMed]
- Marlow, L.A.; D’Innocenzi, J.; Zhang, Y.; Rohl, S.D.; Cooper, S.J.; Sebo, T.; Grant, C.; McIver, B.; Kasperbauer, J.L.; Wadsworth, J.T.; et al. Detailed molecular fingerprinting of four new anaplastic thyroid carcinoma cell lines and their use for verification of RhoB as a molecular therapeutic target. J. Clin. Endocrinol. Metab. 2010, 95, 5338–5347. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.S.; Wang, Q.; Ma, J.X.; Yang, X.H.; Wu, M.L.; Zhang, K.L.; Kong, Q.Y.; Chen, X.Y.; Sun, Y.; Chen, N.N.; et al. CRABP-II methylation: A critical determinant of retinoic acid resistance of medulloblastoma cells. Mol. Oncol. 2012, 6, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, H.; Shu, X.H.; Shi, H.; Chen, X.Y.; Kong, Q.Y.; Wu, M.L.; Liu, J. Distinct sulfonation activities in resveratrol-sensitive and resveratrol-insensitive human glioblastoma cells. FEBS J. 2012, 279, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.H.; Li, H.; Sun, Z.; Wu, M.L.; Ma, J.X.; Wang, J.M.; Wang, Q.; Sun, Y.; Fu, Y.S.; Chen, X.Y.; et al. Identification of metabolic pattern and bioactive form of resveratrol in human medulloblastoma cells. Biochem. Pharmacol. 2010, 79, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.N.; Li, Y.; Wu, M.L.; Liu, Z.L.; Fu, Y.S.; Kong, Q.Y.; Chen, X.Y.; Li, H.; Liu, J. CRABP-II- and FABP5-independent all-trans retinoic acid resistance in COLO 16 human cutaneous squamous cancer cells. Exp. Dermatol. 2012, 21, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Li, P.N.; Li, H.; Wu, M.L.; Wang, S.Y.; Kong, Q.Y.; Zhang, Z.; Sun, Y.; Liu, J.; Lv, D.C. A cost-effective transparency-based digital imaging for efficient and accurate wound area measurement. PLoS ONE 2012, 7, e38069. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Simeone, P.; Toraldo, D.; Del Boccio, P.; Vergaro, V.; Leporatti, S.; Pieragostino, D.; Tinelli, A.; De Domenico, S.; Alberti, S.; et al. Resveratrol downregulates Akt/GSK and ERK signalling pathways in OVCAR-3 ovarian cancer cells. Mol. Biosyst. 2012, 8, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, H.; Zhang, P.; Yu, L.J.; Huang, T.M.; Song, X.; Kong, Q.Y.; Dong, J.L.; Li, P.N.; Liu, J. SHP2, SOCS3 and PIAS3 expression patterns in medulloblastomas: Relevance to STAT3 activation and resveratrol-suppressed STAT3 signaling. Nutrients 2016, 9, 3. [Google Scholar] [CrossRef] [PubMed]
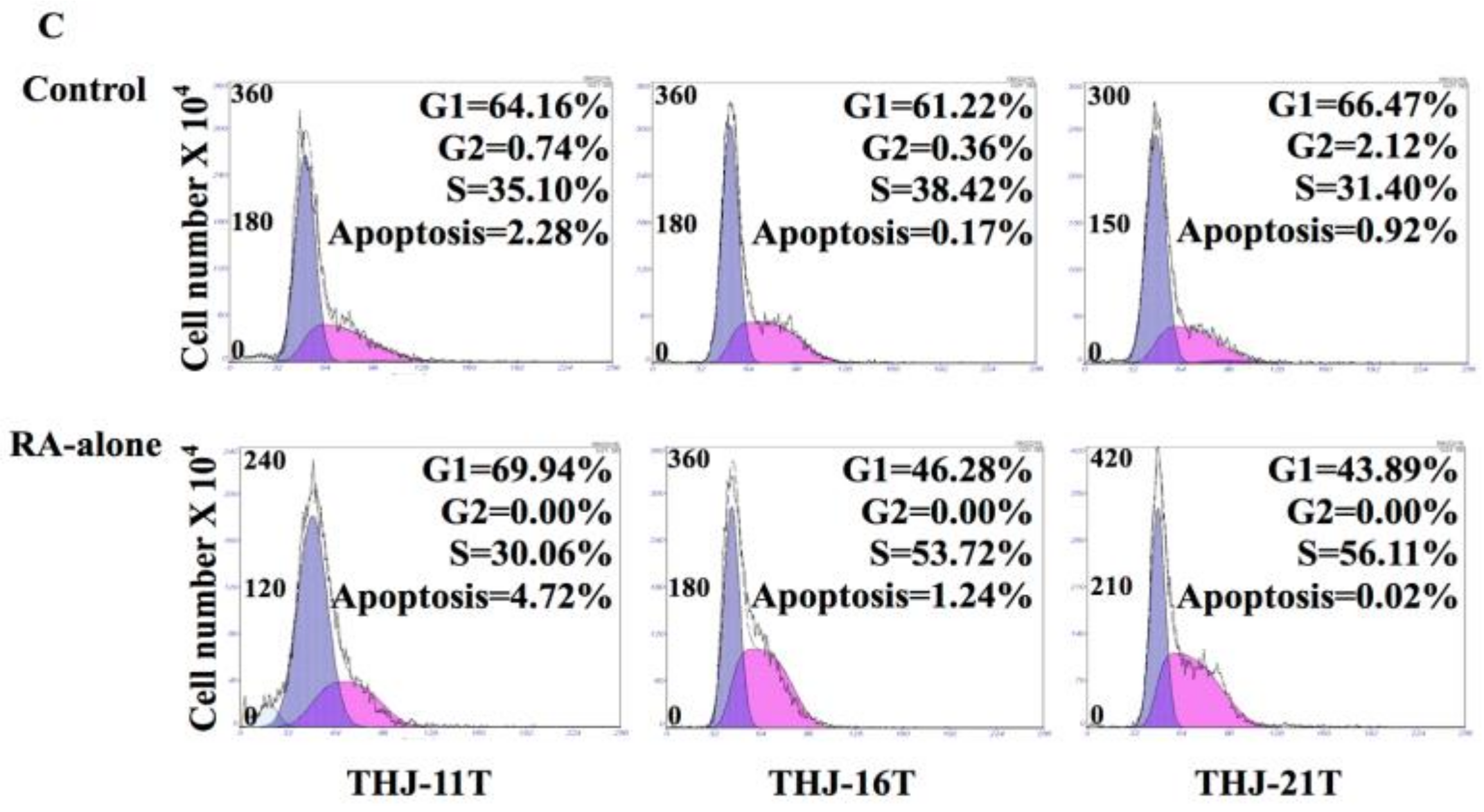
 , apoptosis peak;
, apoptosis peak;  , G1 phase;
, G1 phase;  , S phase;
, S phase;  , G2 phase.
, G2 phase.
 , apoptosis peak;
, apoptosis peak;  , G1 phase;
, G1 phase;  , S phase;
, S phase;  , G2 phase.
, G2 phase.
 , apoptosis peak;
, apoptosis peak;  , G1 phase;
, G1 phase;  , S phase;
, S phase;  , G2 phase.
, G2 phase.
 , apoptosis peak;
, apoptosis peak;  , G1 phase;
, G1 phase;  , S phase;
, S phase;  , G2 phase.
, G2 phase.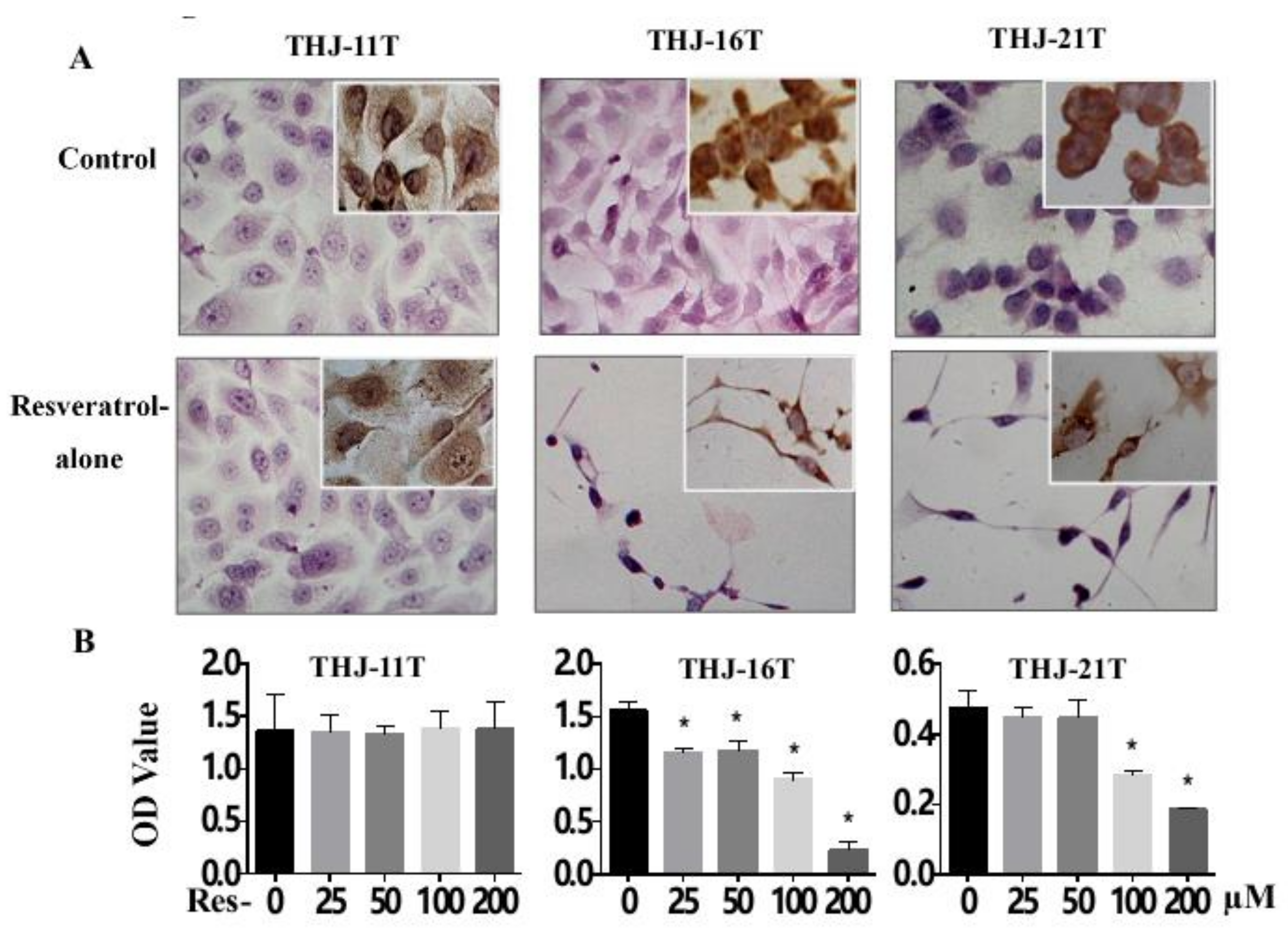

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-T.; Tian, X.-T.; Wu, M.-L.; Zheng, X.; Kong, Q.-Y.; Cheng, X.-X.; Zhu, G.-W.; Liu, J.; Li, H. Resveratrol Suppresses the Growth and Enhances Retinoic Acid Sensitivity of Anaplastic Thyroid Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1030. https://doi.org/10.3390/ijms19041030
Li Y-T, Tian X-T, Wu M-L, Zheng X, Kong Q-Y, Cheng X-X, Zhu G-W, Liu J, Li H. Resveratrol Suppresses the Growth and Enhances Retinoic Acid Sensitivity of Anaplastic Thyroid Cancer Cells. International Journal of Molecular Sciences. 2018; 19(4):1030. https://doi.org/10.3390/ijms19041030
Chicago/Turabian StyleLi, Yi-Tian, Xiao-Ting Tian, Mo-Li Wu, Xu Zheng, Qing-You Kong, Xiao-Xin Cheng, Guang-Wen Zhu, Jia Liu, and Hong Li. 2018. "Resveratrol Suppresses the Growth and Enhances Retinoic Acid Sensitivity of Anaplastic Thyroid Cancer Cells" International Journal of Molecular Sciences 19, no. 4: 1030. https://doi.org/10.3390/ijms19041030