Insulin-Like Growth Factor-1 as a Possible Alternative to Bone Morphogenetic Protein-7 to Induce Osteogenic Differentiation of Human Mesenchymal Stem Cells in Vitro
Abstract
:1. Introduction
2. Results
2.1. Cellular Characteristics and Morphology
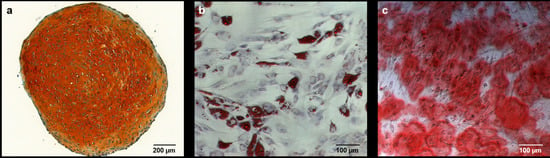
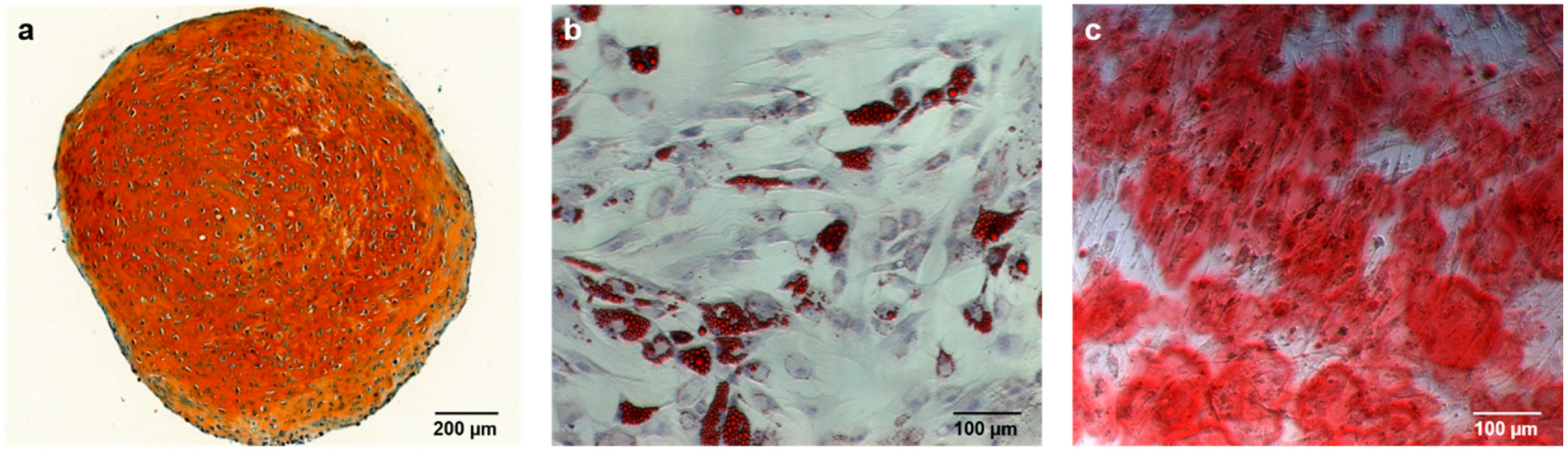
2.2. Alizarin Red Staining
2.2.1. Comparison to Control Group
2.2.2. Comparison of IGF-1 to BMP-7
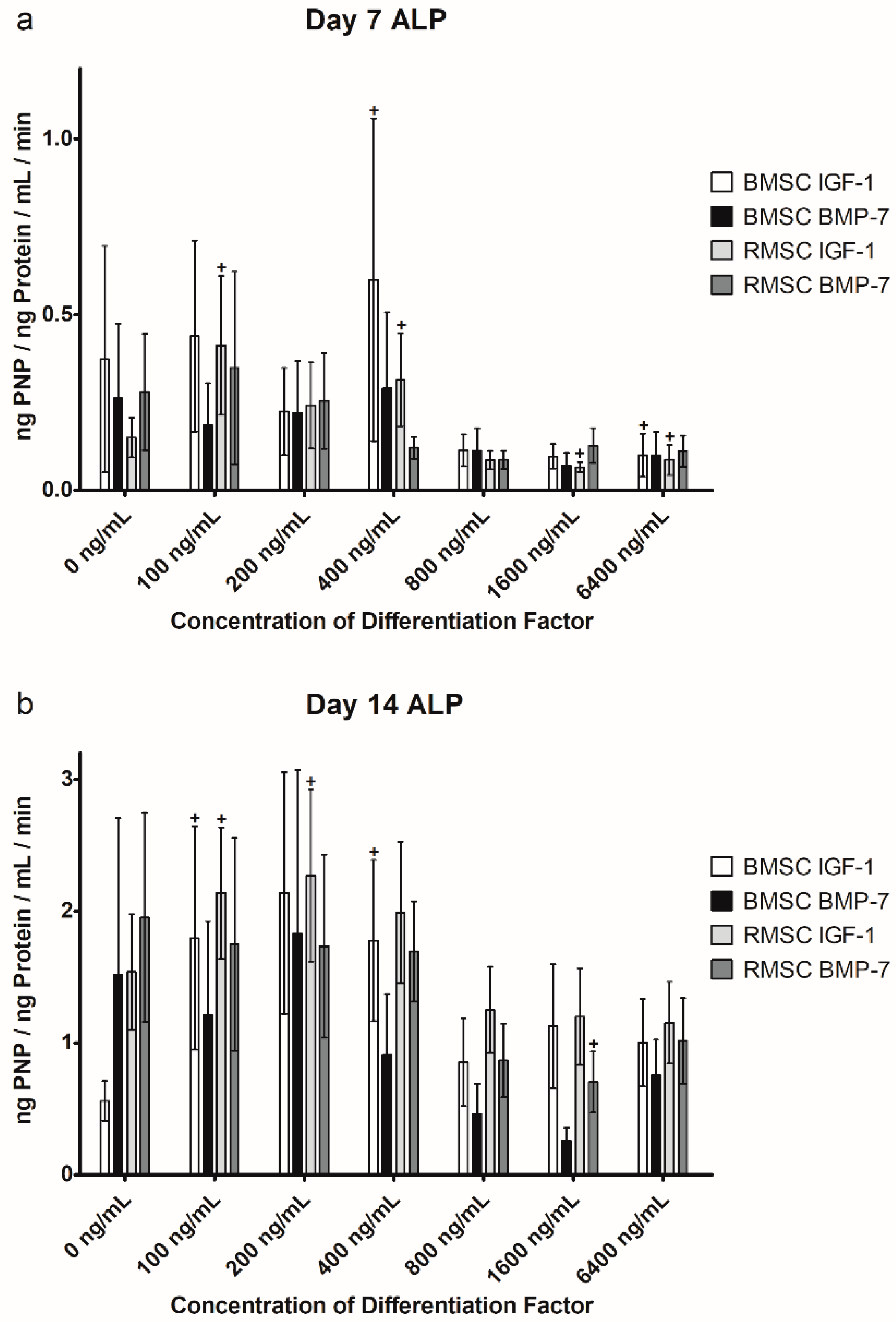
2.3. Alkaline Phosphatase (ALP) Activity
2.3.1. Comparison to Control Group
2.3.2. Comparison of IGF-1 to BMP-7
3. Discussion
4. Materials and Methods
4.1. Donor Patients
4.2. Primary Cell Culture and Isolation of Mesenchymal Stem Cells
4.3. Osteogenic Differentiation
4.4. Quantification of Osteogenic Differentiation
4.5. Statistics
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| ATSC | Adipose tissue derived mesenchymal stem cells |
| BMP-7 | Bone morphogenetic protein 7 |
| BMSC | Mesenchymal stem cells harvested via iliac crest bone marrow aspiration |
| BSA | Bovine serum albumin |
| CD | Cluster of differentiation |
| DMEM | Dulbecco’s modified eagle medium |
| FCS | Fetal calf serum |
| IGF-1 | Insulin-like growth factor 1 |
| MSC | human mesenchymal stem cells |
| ODM | Osteogenic differentiation medium |
| PNP | Para-Nitrophenol |
| PNPP | Para-Nitrophenylphosphate |
| RMSC | Mesenchymal stem cells harvested via Reamer-Irrigator-Aspirator |
| RUNX2 | Runt-related transcription factor 2 |
| TGF-β1 | Transforming growth factor β1 |
References
- Hoellig, M.; Westhauser, F.; Kornienko, K.; Xiao, K.; Schmidmaier, G.; Moghaddam, A. Mesenchymal stem cells from reaming material possess high osteogenic potential and react sensitively to bone morphogenetic protein 7. J. Appl. Biomater. Funct. Mater. 2017, 15, e54–e62. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38 (Suppl. 4), S3–S6. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A.; Schmidmaier, G.; Marsh, D. The diamond concept—Open questions. Injury 2008, 39 (Suppl. 2), S5–S8. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavery, K.; Swain, P.; Falb, D.; Alaoui-Ismaili, M.H. BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J. Biol. Chem. 2008, 283, 20948–20958. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Bruder, S.P. Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends Mol. Med. 2001, 7, 259–264. [Google Scholar] [CrossRef]
- Kuehlfluck, P.; Moghaddam, A.; Helbig, L.; Child, C.; Wildemann, B.; Schmidmaier, G.; Group, H.T.-H.T.R. RIA fractions contain mesenchymal stroma cells with high osteogenic potency. Injury 2015, 46 (Suppl. 8), S23–S32. [Google Scholar] [CrossRef]
- Westhauser, F.; Hollig, M.; Reible, B.; Xiao, K.; Schmidmaier, G.; Moghaddam, A. Bone formation of human mesenchymal stem cells harvested from reaming debris is stimulated by low-dose bone morphogenetic protein-7 application in vivo. J. Orthop. 2016, 13, 404–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of iliac crest bone graft harvesting. Clin. Orthop. Relat. Res. 1996, 329, 300–309. [Google Scholar] [CrossRef]
- Cox, G.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Reamer-irrigator-aspirator indications and clinical results: A systematic review. Int. Orthop. 2011, 35, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Wenisch, S.; Trinkaus, K.; Hild, A.; Hose, D.; Herde, K.; Heiss, C.; Kilian, O.; Alt, V.; Schnettler, R. Human reaming debris: A source of multipotent stem cells. Bone 2005, 36, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Chen, C.; Pang, X.; Uludag, H.; Jiang, H. Synergistic effect of recombinant human bone morphogenic protein-7 and osteogenic differentiation medium on human bone-marrow-derived mesenchymal stem cells in vitro. Int. Orthop. 2011, 35, 1889–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, J.C.; Strohbach, C.A.; Wenke, J.C.; Rathbone, C.R. Beyond osteogenesis: An in vitro comparison of the potentials of six bone morphogenetic proteins. Front. Pharmacol. 2013, 4, 125. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.; Elleser, C.; Biglari, B.; Wentzensen, A.; Zimmermann, G. Clinical application of BMP 7 in long bone non-unions. Arch. Orthop. Trauma Surg. 2010, 130, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.; Moghaddam, A.; Wagner, C.; Vock, B.; Wentzensen, A. Clinical experience with bone morphogenetic protein 7 (BMP 7) in nonunions of long bones. Unfallchirurg 2006, 109, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G.; Muller, U.; Loffler, C.; Wentzensen, A.; Moghaddam, A. Therapeutic outcome in tibial pseudarthrosis: Bone morphogenetic protein 7 (BMP-7) versus autologous bone grafting for tibial fractures. Unfallchirurg 2007, 110, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Hackl, S.; Hierholzer, C.; Friederichs, J.; Woltmann, A.; Buhren, V.; von Ruden, C. Long-term outcome following additional rhBMP-7 application in revision surgery of aseptic humeral, femoral, and tibial shaft nonunion. BMC Musculoskelet. Disord. 2017, 18, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghaddam, A.; Zietzschmann, S.; Bruckner, T.; Schmidmaier, G. Treatment of atrophic tibia non-unions according to “diamond concept”: Results of one- and two-step treatment. Injury 2015, 46 (Suppl. 4), S39–S50. [Google Scholar] [CrossRef]
- Moghaddam-Alvandi, A.; Zimmermann, G.; Buchler, A.; Elleser, C.; Biglari, B.; Grutzner, P.A.; Wolfl, C.G. Results of nonunion treatment with bone morphogenetic protein 7 (BMP-7). Unfallchirurg 2012, 115, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Garrison, K.R.; Shemilt, I.; Donell, S.; Ryder, J.J.; Mugford, M.; Harvey, I.; Song, F.; Alt, V. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst. Rev. 2010, CD006950. [Google Scholar] [CrossRef] [PubMed]
- Lissenberg-Thunnissen, S.N.; de Gorter, D.J.; Sier, C.F.; Schipper, I.B. Use and efficacy of bone morphogenetic proteins in fracture healing. Int. Orthop. 2011, 35, 1271–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, G.B.; Einhorn, T.A. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int. Orthop. 2007, 31, 721–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reible, B.; Schmidmaier, G.; Prokscha, M.; Moghaddam, A.; Westhauser, F. Continuous stimulation with differentiation factors is necessary to enhance osteogenic differentiation of human mesenchymal stem cells in vitro. Growth Factors 2017, 35, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Westhauser, F.; Zimmermann, G.; Moghaddam, S.; Bruckner, T.; Schmidmaier, G.; Biglari, B.; Moghaddam, A. Reaming in treatment of non-unions in long bones: Cytokine expression course as a tool for evaluation of non-union therapy. Arch. Orthop. Trauma Surg. 2015, 135, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E. Insulin like growth factors and the local regulation of bone formation. Bone 1993, 14, 273–276. [Google Scholar] [CrossRef]
- Han, V.K.; D’Ercole, A.J.; Lund, P.K. Cellular localization of somatomedin (insulin-like growth factor) messenger RNA in the human fetus. Science 1987, 236, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Bondy, C.A.; Werner, H.; Roberts, C.T.J.; LeRoith, D. Cellular pattern of insulin-like growth factor-I (IGF-I) and type I IGF receptor gene expression in early organogenesis: Comparison with IGF-II gene expression. Mol. Endocrinol. 1990, 4, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Edwall, D.; Prisell, P.T.; Levinovitz, A.; Jennische, E.; Norstedt, G. Expression of insulin-like growth factor I messenger ribonucleic acid in regenerating bone after fracture: Influence of indomethacin. J. Bone Min. Res. 1992, 7, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Jennische, E.; Matejka, G.L. IGF-I binding and IGF-I expression in regenerating muscle of normal and hypophysectomized rats. Acta Physiol. Scand. 1992, 146, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Tiangco, D.A.; Papakonstantinou, K.C.; Mullinax, K.A.; Terzis, J.K. IGF-I and end-to-side nerve repair: A dose-response study. J. Reconstr. Microsurg. 2001, 17, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Konturek, S.J.; Tomaszewska, R.; Stachura, J.; Konturek, P.C. IGF-1 stimulates production of interleukin-10 and inhibits development of caerulein-induced pancreatitis. J. Physiol. Pharmacol. 2003, 54, 575–590. [Google Scholar] [PubMed]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Konturek, S.J.; Polus, A.; Pawlik, W.W.; Kuwahara, A.; Kato, I.; et al. Influence of ghrelin on gastric and duodenal growth and expression of digestive enzymes in young mature rats. J. Physiol. Pharmacol. 2006, 57, 425–437. [Google Scholar] [PubMed]
- Frascarelli, S.; Ghelardoni, S.; Ronca-Testoni, S.; Zucchi, R. Effect of ghrelin and synthetic growth hormone secretagogues in normal and ischemic rat heart. Basic Res. Cardiol. 2003, 98, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Nishimatsu, H.; Suzuki, E.; Satonaka, H.; Nagata, D.; Oba, S.; Sata, M.; Takahashi, M.; Yamamoto, Y.; Terauchi, Y.; et al. Ghrelin improves renal function in mice with ischemic acute renal failure. J. Am. Soc. Nephrol. 2006, 17, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, C.; Meng, B.; Tang, T.; Shi, Q.; Yang, H. Acute effect of Ghrelin on ischemia/reperfusion injury in the rat spinal cord. Int. J. Mol. Sci. 2012, 13, 9864–9876. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Ceranowicz, P.; Dembinski, A.; Cieszkowski, J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Kuwahara, A.; Kato, I. Therapeutic effect of ghrelin in the course of cerulein-induced acute pancreatitis in rats. J. Physiol. Pharmacol. 2010, 61, 419–427. [Google Scholar] [PubMed]
- Ceranowicz, D.; Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Cieszkowski, J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Kuwahara, A.; Kato, I. Role of hormonal axis, growth hormone—IGF-1, in the therapeutic effect of ghrelin in the course of cerulein-induced acute pancreatitis. J. Physiol. Pharmacol. 2010, 61, 599–606. [Google Scholar] [PubMed]
- Bukowczan, J.; Warzecha, Z.; Ceranowicz, P.; Kusnierz-Cabala, B.; Tomaszewska, R.; Dembinski, A. Therapeutic effect of ghrelin in the course of ischemia/reperfusion-induced acute pancreatitis. Curr. Pharm. Des. 2015, 21, 2284–2290. [Google Scholar] [CrossRef] [PubMed]
- Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Kusnierz-Cabala, B.; Bonior, J.; Jaworek, J.; Ambrozy, T.; Gil, K.; Olszanecki, R.; et al. Essential Role of Growth Hormone and IGF-1 in Therapeutic Effect of Ghrelin in the Course of Acetic Acid-Induced Colitis. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Galazka, K.; Bonior, J.; Jaworek, J.; Bartus, K.; Gil, K.; et al. Exogenous Ghrelin Accelerates the Healing of Acetic Acid-Induced Colitis in Rats. Int. J. Mol. Sci. 2016, 17, 1455. [Google Scholar] [CrossRef] [PubMed]
- Cieszkowski, J.; Warzecha, Z.; Ceranowicz, P.; Ceranowicz, D.; Kusnierz-Cabala, B.; Pedziwiatr, M.; Dembinski, M.; Ambrozy, T.; Kaczmarzyk, T.; Pihut, M.; et al. Therapeutic effect of exogenous ghrelin in the healing of gingival ulcers is mediated by the release of endogenous growth hormone and insulin-like growth factor-1. J. Physiol. Pharmacol. 2017, 68, 609–617. [Google Scholar] [PubMed]
- Abdanipour, A.; Shahsavandi, B.; Alipour, M.; Feizi, H. Ghrelin Upregulates Hoxb4 Gene Expression in Rat Bone Marrow Stromal Cells. Cell J. 2018, 20, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Schmidmaier, G.; Wildemann, B.; Heeger, J.; Gabelein, T.; Flyvbjerg, A.; Bail, H.J.; Raschke, M. Improvement of fracture healing by systemic administration of growth hormone and local application of insulin-like growth factor-1 and transforming growth factor-beta1. Bone 2002, 31, 165–172. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Wildemann, B.; Bail, H.; Lucke, M.; Fuchs, T.; Stemberger, A.; Flyvbjerg, A.; Haas, N.P.; Raschke, M. Local application of growth factors (insulin-like growth factor-1 and transforming growth factor-beta1) from a biodegradable poly(D,L-lactide) coating of osteosynthetic implants accelerates fracture healing in rats. Bone 2017, 28, 341–350. [Google Scholar] [CrossRef]
- Bernstein, A.; Mayr, H.O.; Hube, R. Can bone healing in distraction osteogenesis be accelerated by local application of IGF-1 and TGF-beta1? J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Schmidmaier, G.; Lucke, M.; Schwabe, P.; Raschke, M.; Haas, N.P.; Wildemann, B. Collective review: Bioactive implants coated with poly(D,L-lactide) and growth factors IGF-I, TGF-beta1, or BMP-2 for stimulation of fracture healing. J. Long Term Eff. Med. Implant. 2006, 16, 61–69. [Google Scholar] [CrossRef]
- Wildemann, B.; Burkhardt, N.; Luebberstedt, M.; Vordemvenne, T.; Schmidmaier, G. Proliferating and differentiating effects of three different growth factors on pluripotent mesenchymal cells and osteoblast like cells. J. Orthop. Surg. Res. 2007, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Granero-Molto, F.; Myers, T.J.; Weis, J.A.; Longobardi, L.; Li, T.; Yan, Y.; Case, N.; Rubin, J.; Spagnoli, A. Mesenchymal stem cells expressing insulin-like growth factor-I (MSCIGF) promote fracture healing and restore new bone formation in Irs1 knockout mice: Analyses of MSCIGF autocrine and paracrine regenerative effects. Stem Cells 2011, 29, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.J.; Yan, Y.; Granero-Molto, F.; Weis, J.A.; Longobardi, L.; Li, T.; Li, Y.; Contaldo, C.; Ozkan, H.; Spagnoli, A. Systemically delivered insulin-like growth factor-I enhances mesenchymal stem cell-dependent fracture healing. Growth Factors 2012, 30, 230–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, C.; Doll, J.; Tanner, M.; Bruckner, T.; Zimmermann, G.; Helbig, L.; Biglari, B.; Schmidmaier, G.; Moghaddam, A. Quantification of TGF-ss1, PDGF and IGF-1 cytokine expression after fracture treatment vs. non-union therapy via masquelet. Injury 2016, 47, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Henle, P.; Bidlingmaier, M.; Moghaddam, A.; Kasten, P.; Zimmermann, G. Systemic response of the GH/IGF-I axis in timely versus delayed fracture healing. Growth Horm. IGF Res. 2008, 18, 205–212. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Balhara, B.; Misra, M.; Levitsky, L.L. Recombinant human IGF-1 (Insulin-Like Growth Factor) therapy: Where do we stand today? Indian J. Pediatr. 2012, 79, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Jadlowiec, J.A.; Campbell, P.G. Insulin-like growth factor-I induces early osteoblast gene expression in human mesenchymal stem cells. Stem Cells Dev. 2005, 14, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Schmidmaier, G.; Wildemann, B.; Gabelein, T.; Heeger, J.; Kandziora, F.; Haas, N.P.; Raschke, M. Synergistic effect of IGF-I and TGF-beta1 on fracture healing in rats: Single versus combined application of IGF-I and TGF-beta1. Acta Orthop. Scand. 2003, 74, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Aguiar, D.J.; Williams, S.M.; La Pean, A.; Pan, W.; Verfaillie, C.M. Identification of genes responsible for osteoblast differentiation from human mesodermal progenitor cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3305–3310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Tzioupis, C. Clinical applications of BMP-7: The UK perspective. Injury 2005, 36 (Suppl. 3), S47–S50. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, R.; Stromqvist, B.; Aspenberg, P. Randomized radiostereometric study comparing osteogenic protein-1 (BMP-7) and autograft bone in human noninstrumented posterolateral lumbar fusion: 2002 Volvo Award in clinical studies. Spine 2002, 27, 2654–2661. [Google Scholar] [CrossRef] [PubMed]


| MSC-Subtype | CD90 | CD73 | CD105 | Negative Control |
|---|---|---|---|---|
| BMSC | 95.23% (0.88%) | 99.97% (0.01%) | 99.88% (0.05%) | 0.51% (0.12%) |
| RMSC | 96.49% (0.93%) | 99.99% (<0.01%) | 99.92% (0.04%) | 0.63% (0.11%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reible, B.; Schmidmaier, G.; Moghaddam, A.; Westhauser, F. Insulin-Like Growth Factor-1 as a Possible Alternative to Bone Morphogenetic Protein-7 to Induce Osteogenic Differentiation of Human Mesenchymal Stem Cells in Vitro. Int. J. Mol. Sci. 2018, 19, 1674. https://doi.org/10.3390/ijms19061674
Reible B, Schmidmaier G, Moghaddam A, Westhauser F. Insulin-Like Growth Factor-1 as a Possible Alternative to Bone Morphogenetic Protein-7 to Induce Osteogenic Differentiation of Human Mesenchymal Stem Cells in Vitro. International Journal of Molecular Sciences. 2018; 19(6):1674. https://doi.org/10.3390/ijms19061674
Chicago/Turabian StyleReible, Bruno, Gerhard Schmidmaier, Arash Moghaddam, and Fabian Westhauser. 2018. "Insulin-Like Growth Factor-1 as a Possible Alternative to Bone Morphogenetic Protein-7 to Induce Osteogenic Differentiation of Human Mesenchymal Stem Cells in Vitro" International Journal of Molecular Sciences 19, no. 6: 1674. https://doi.org/10.3390/ijms19061674