Scorpins in the DNA Damage Response
Abstract
:1. Introduction
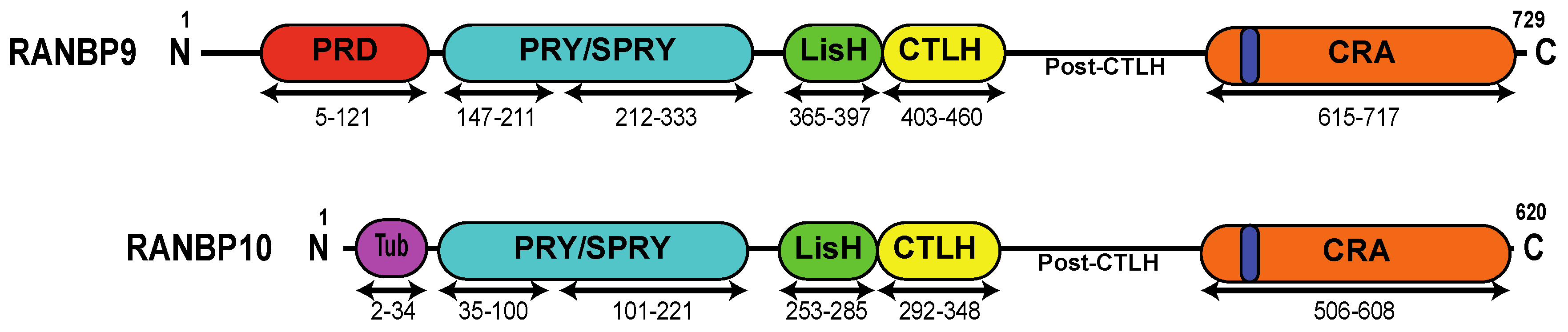
2. Scorpins
3. Known Biological Roles of RANBP9
4. RANBP9 in the DDR
4.1. RANBP9 and Sensitivity to Ionizing Radiation (IR)
4.2. RANBP9 and Post-Translational Modifications Following Stress
4.3. RANBP9 Protein-Protein Interactions Relevant to the DDR
4.4. RANBP9 as a Target and Signaling Facilitator of the Ataxia Telangiectasia Mutated (ATM) Kinase
4.5. RANBP9 as Pro-Apoptotic Tumor Suppressor
5. Known Biological Roles of RANBP10
6. RANBP10 in the DDR
7. Future Perspectives
Funding
Conflicts of Interest
References
- Desai, A.; Yan, Y.; Gerson, S.L. Advances in therapeutic targeting of the DNA damage response in cancer. DNA Repair. (Amst) 2018, 66–67, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Postel-Vinay, S.; Vanhecke, E.; Olaussen, K.A.; Lord, C.J.; Ashworth, A.; Soria, J.C. The potential of exploiting DNA-repair defects for optimizing lung cancer treatment. Nat. Rev. Clin. oncol. 2012, 9, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Helena, J.M.; Joubert, A.M.; Grobbelaar, S.; Nolte, E.M.; Nel, M.; Pepper, M.S.; Coetzee, M.; Mercier, A.E. Deoxyribonucleic Acid Damage and Repair: Capitalizing on Our Understanding of the Mechanisms of Maintaining Genomic Integrity for Therapeutic Purposes. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. BRCAness revisited. Nat. Rev. Cancer 2016, 16, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Mouw, K.W.; Goldberg, M.S.; Konstantinopoulos, P.A.; D’Andrea, A.D. DNA Damage and Repair Biomarkers of Immunotherapy Response. Cancer Discov. 2017, 7, 675–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salemi, L.M.; Maitland, M.E.R.; McTavish, C.J.; Schild-Poulter, C. Cell signalling pathway regulation by RanBPM: Molecular insights and disease implications. Open Biol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Suresh, B.; Ramakrishna, S.; Baek, K.H. Diverse roles of the scaffolding protein RanBPM. Drug Discov. Today 2012, 17, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Puverel, S.; Tessarollo, L. RanBPM, a scaffolding protein for gametogenesis. Curr. Top. Dev. Biol. 2013, 102, 357–384. [Google Scholar] [CrossRef] [PubMed]
- Murrin, L.C.; Talbot, J.N. RanBPM, a scaffolding protein in the immune and nervous systems. J. Neuroimmune Pharmacol. 2007, 2, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Schulze, H.; Dose, M.; Korpal, M.; Meyer, I.; Italiano, J.E., Jr.; Shivdasani, R.A. RanBP10 is a cytoplasmic guanine nucleotide exchange factor that modulates noncentrosomal microtubules. J. Biol. Chem. 2008, 283, 14109–14119. [Google Scholar] [CrossRef] [PubMed]
- Flaumenhaft, R. Getting in shape with RanBP10. Blood 2009, 114, 5412–5413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, I.; Kunert, S.; Schwiebert, S.; Hagedorn, I.; Italiano, J.E., Jr.; Dutting, S.; Nieswandt, B.; Bachmann, S.; Schulze, H. Altered microtubule equilibrium and impaired thrombus stability in mice lacking RanBP10. Blood 2012, 120, 3594–3602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M.; Masuda, H.; Horii, J.; Kuma, K.; Yokoyama, N.; Ohba, T.; Nishitani, H.; Miyata, T.; Tanaka, M.; Nishimoto, T. When overexpressed, a novel centrosomal protein, RanBPM, causes ectopic microtubule nucleation similar to gamma-tubulin. J. Cell Biol. 1998, 143, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, H.; Hirose, E.; Uchimura, Y.; Nakamura, M.; Umeda, M.; Nishii, K.; Mori, N.; Nishimoto, T. Full-sized RanBPM cDNA encodes a protein possessing a long stretch of proline and glutamine within the N-terminal region, comprising a large protein complex. Gene 2001, 272, 25–33. [Google Scholar] [CrossRef]
- Wang, D.; Li, Z.; Schoen, S.R.; Messing, E.M.; Wu, G. A novel MET-interacting protein shares high sequence similarity with RanBPM, but fails to stimulate MET-induced Ras/Erk signaling. Biochem. Biophys. Res. Commun. 2004, 313, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, T. A new role of ran GTPase. Biochem. Biophys. Res. Commun. 1999, 262, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Hosono, K.; Noda, S.; Shimizu, A.; Nakanishi, N.; Ohtsubo, M.; Shimizu, N.; Minoshima, S. YPEL5 protein of the YPEL gene family is involved in the cell cycle progression by interacting with two distinct proteins RanBPM and RanBP10. Genomics 2010, 96, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Suresh, B.; Kim, H.H.; Ramakrishna, S. RanBPM: A potential therapeutic target for modulating diverse physiological disorders. Drug Discov. Today 2017, 22, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Haq, S.; Ramakrishna, S. Scaffolding protein RanBPM and its interactions in diverse signaling pathways in health and disease. Discov. Med. 2018, 25, 177–194. [Google Scholar] [PubMed]
- Puverel, S.; Barrick, C.; Dolci, S.; Coppola, V.; Tessarollo, L. RanBPM is essential for mouse spermatogenesis and oogenesis. Development 2011, 138, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.; Ozaki, T.; Miyazaki, K.; Kato, C.; Hanamoto, T.; Nakagawara, A. Protein stability and function of p73 are modulated by a physical interaction with RanBPM in mammalian cultured cells. Oncogene 2005, 24, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Suresh, B.; Ramakrishna, S.; Kim, Y.S.; Kim, S.M.; Kim, M.S.; Baek, K.H. Stability and function of mammalian lethal giant larvae-1 oncoprotein are regulated by the scaffolding protein RanBPM. J. Biol. Chem. 2010, 285, 35340–35349. [Google Scholar] [CrossRef] [PubMed]
- Atabakhsh, E.; Schild-Poulter, C. RanBPM is an inhibitor of ERK signaling. PLoS ONE 2012, 7, e47803. [Google Scholar] [CrossRef] [PubMed]
- Palavicini, J.P.; Wang, H.; Minond, D.; Bianchi, E.; Xu, S.; Lakshmana, M.K. RanBP9 overexpression down-regulates phospho-cofilin, causes early synaptic deficits and impaired learning, and accelerates accumulation of amyloid plaques in the mouse brain. J. Alzheimers Dis. 2014, 39, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, D.; Scarpa, M.; Tessari, A.; Uka, R.; Amari, F.; Lee, C.; Richmond, T.; Foray, C.; Sheetz, T.; Braddom, A.; et al. Ran Binding Protein 9 (RanBP9) is a novel mediator of cellular DNA damage response in lung cancer cells. Oncotarget 2016, 7, 18371–18383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puverel, S.; Kiris, E.; Singh, S.; Klarmann, K.D.; Coppola, V.; Keller, J.R.; Tessarollo, L. RanBPM (RanBP9) regulates mouse c-Kit receptor level and is essential for normal development of bone marrow progenitor cells. Oncotarget 2016, 7, 85109–85123. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Suresh, B.; Park, J.H.; Kim, Y.S.; Ramakrishna, S.; Baek, K.H. Ubiquitin-specific protease 11 functions as a tumor suppressor by modulating Mgl-1 protein to regulate cancer cell growth. Oncotarget 2016, 7, 14441–14457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
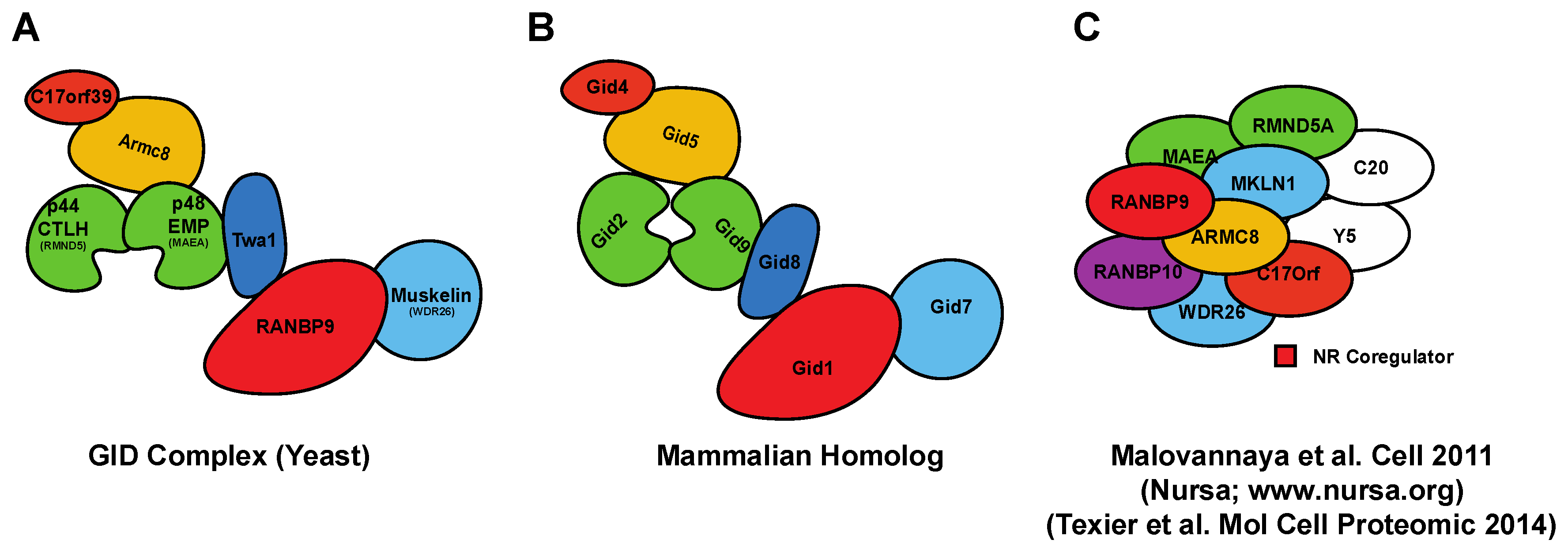
- Malovannaya, A.; Lanz, R.B.; Jung, S.Y.; Bulynko, Y.; Le, N.T.; Chan, D.W.; Ding, C.; Shi, Y.; Yucer, N.; Krenciute, G.; et al. Analysis of the human endogenous coregulator complexome. Cell 2011, 145, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Texier, Y.; Toedt, G.; Gorza, M.; Mans, D.A.; van Reeuwijk, J.; Horn, N.; Willer, J.; Katsanis, N.; Roepman, R.; Gibson, T.J.; et al. Elution profile analysis of SDS-induced subcomplexes by quantitative mass spectrometry. Mol. Cell. Proteomics 2014, 13, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Menssen, R.; Schweiggert, J.; Schreiner, J.; Kusevic, D.; Reuther, J.; Braun, B.; Wolf, D.H. Exploring the topology of the Gid complex, the E3 ubiquitin ligase involved in catabolite-induced degradation of gluconeogenic enzymes. J. Biol. Chem. 2012, 287, 25602–25614. [Google Scholar] [CrossRef] [PubMed]
- Santt, O.; Pfirrmann, T.; Braun, B.; Juretschke, J.; Kimmig, P.; Scheel, H.; Hofmann, K.; Thumm, M.; Wolf, D.H. The yeast GID complex, a novel ubiquitin ligase (E3) involved in the regulation of carbohydrate metabolism. Mol. Biol. Cell 2008, 19, 3323–3333. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.R.; McCann, J.A.; Chiang, H.L. The heat shock protein Ssa2p is required for import of fructose-1, 6-bisphosphatase into Vid vesicles. J. Cell Biol. 2000, 150, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Alibhoy, A.A.; Giardina, B.J.; Dunton, D.D.; Chiang, H.L. Vid30 is required for the association of Vid vesicles and actin patches in the vacuole import and degradation pathway. Autophagy 2012, 8, 29–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yard, B.D.; Adams, D.J.; Chie, E.K.; Tamayo, P.; Battaglia, J.S.; Gopal, P.; Rogacki, K.; Pearson, B.E.; Phillips, J.; Raymond, D.P.; et al. A genetic basis for the variation in the vulnerability of cancer to DNA damage. Nat. Commun. 2016, 7, 11428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denti, S.; Sirri, A.; Cheli, A.; Rogge, L.; Innamorati, G.; Putignano, S.; Fabbri, M.; Pardi, R.; Bianchi, E. RanBPM is a phosphoprotein that associates with the plasma membrane and interacts with the integrin LFA-1. J. Biol. Chem. 2004, 279, 13027–13034. [Google Scholar] [CrossRef] [PubMed]
- Salemi, L.M.; Loureiro, S.O.; Schild-Poulter, C. Characterization of RanBPM molecular determinants that control its subcellular localization. PLoS ONE 2015, 10, e0117655. [Google Scholar] [CrossRef] [PubMed]
- Leu, J.D.; Chiu, Y.W.; Lo, C.C.; Chiang, P.H.; Chiu, S.J.; Tsai, C.H.; Hwang, J.J.; Chen, R.C.; Gorbunova, V.; Lee, Y.J. Enhanced cellular radiosensitivity induced by cofilin-1 over-expression is associated with reduced DNA repair capacity. Int. J. Radiat. Biol. 2013, 89, 433–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.Y.; Leu, J.D.; Lee, Y.J. The actin depolymerizing factor (ADF)/cofilin signaling pathway and DNA damage responses in cancer. Int. J. Mol. Sci. 2015, 16, 4095–4120. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.A.; Boggess, T.; Uhlar, C.; Wang, X.; Khan, H.; Cappos, G.; Joly-Amado, A.; De Narvaez, E.; Majid, S.; Minamide, L.S.; et al. RanBP9 at the intersection between cofilin and Abeta pathologies: Rescue of neurodegenerative changes by RanBP9 reduction. Cell Death Dis. 2015, 6, 1676. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K. Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell Signal. 2013, 25, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.E.; Woo, J.A.; Lakshmana, M.K.; Uhlar, C.; Ankala, V.; Boggess, T.; Liu, T.; Hong, Y.H.; Mook-Jung, I.; Kim, S.J.; et al. Mitochondrial dysfunction and calcium deregulation by the RanBP9-cofilin pathway. FASEB J. 2013, 27, 4776–4789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, J.A.; Jung, A.R.; Lakshmana, M.K.; Bedrossian, A.; Lim, Y.; Bu, J.H.; Park, S.A.; Koo, E.H.; Mook-Jung, I.; Kang, D.E. Pivotal role of the RanBP9-cofilin pathway in Abeta-induced apoptosis and neurodegeneration. Cell Death Differ. 2012, 19, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, X.; Hu, G.; Li, X.; Zhuang, Z.; Liu, J.; Wu, D.; Yang, L.; Xu, X.; Huang, X.; et al. Poly(ADP-ribose) glycohydrolase silencing down-regulates TCTP and Cofilin-1 associated with metastasis in benzo(a)pyrene carcinogenesis. Am. J. Cancer Res. 2015, 5, 155–167. [Google Scholar] [PubMed]
- Lee, Y.J.; Sheu, T.J.; Keng, P.C. Enhancement of radiosensitivity in H1299 cancer cells by actin-associated protein cofilin. Biochem. Biophys. Res. Commun. 2005, 335, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Marion Schneider, E.; Li, X.; Duttenhofer, I.; Debatin, K.; Hug, H. HIPK2 associates with RanBPM. Biochem. Biophys. Res. Commun. 2002, 297, 148–153. [Google Scholar] [CrossRef]
- Hofmann, T.G.; Glas, C.; Bitomsky, N. HIPK2: A tumour suppressor that controls DNA damage-induced cell fate and cytokinesis. Bioessays 2013, 35, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Roh, S.E.; Woo, J.A.; Ryu, H.; Kang, D.E. Cooperative role of RanBP9 and P73 in mitochondria-mediated apoptosis. Cell Death Dis. 2013, 4, e476. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Sengupta, S.; Gurdziel, K.; Bell, G.W.; Jacks, T.; Flores, E.R. p63 and p73 transcriptionally regulate genes involved in DNA repair. PLoS Genet. 2009, 5, e1000680. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Yan, G.; Song, X.; Xie, L.; Zhou, Y.; Li, J.; Hu, X.; Li, Z.; Hu, J.; Zhang, Y.; et al. Deubiquitylation and stabilization of p21 by USP11 is critical for cell-cycle progression and DNA damage responses. Proc. Natl. Acad. Sci. USA 2018, 115, 4678–4683. [Google Scholar] [CrossRef] [PubMed]
- Orthwein, A.; Noordermeer, S.M.; Wilson, M.D.; Landry, S.; Enchev, R.I.; Sherker, A.; Munro, M.; Pinder, J.; Salsman, J.; Dellaire, G.; et al. A mechanism for the suppression of homologous recombination in G1 cells. Nature 2015, 528, 422–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, M.A.; Cheng, H.; Quayle, A.N.; Nishitani, H.; Nelson, C.C.; Rennie, P.S. RanBPM, a nuclear protein that interacts with and regulates transcriptional activity of androgen receptor and glucocorticoid receptor. J. Biol. Chem. 2002, 277, 48020–48027. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Yokoyama, T.; Yamaji, R.; Nakano, Y.; Inui, H. RanBP10 acts as a novel coactivator for the androgen receptor. Biochem. Biophys. Res. Commun. 2008, 368, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.C.; Li, L. Connecting androgen receptor signaling and the DNA damage response: Development of new therapies for advanced prostate cancer. Mol. Cell. Oncol. 2017, 4, e1321167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polkinghorn, W.R.; Parker, J.S.; Lee, M.X.; Kass, E.M.; Spratt, D.E.; Iaquinta, P.J.; Arora, V.K.; Yen, W.F.; Cai, L.; Zheng, D.; et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013, 3, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, M.; Vermezovic, J.; d'Adda di Fagagna, F. NOTCH1 Inhibits Activation of ATM by Impairing the Formation of an ATM-FOXO3a-KAT5/Tip60 Complex. Cell Rep. 2016, 16, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Bhoumik, A.; Singha, N.; O'Connell, M.J.; Ronai, Z.A. Regulation of TIP60 by ATF2 modulates ATM activation. J. Biol. Chem. 2008, 283, 17605–17614. [Google Scholar] [CrossRef] [PubMed]
- Eymin, B.; Claverie, P.; Salon, C.; Leduc, C.; Col, E.; Brambilla, E.; Khochbin, S.; Gazzeri, S. p14ARF activates a Tip60-dependent and p53-independent ATM/ATR/CHK pathway in response to genotoxic stress. Mol. Cell. Biol. 2006, 26, 4339–4350. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Sun, Y.; Chen, S.; Roy, K.; Price, B.D. The FATC domains of PIKK proteins are functionally equivalent and participate in the Tip60-dependent activation of DNA-PKcs and ATM. J. Biol. Chem. 2006, 281, 15741–15746. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, X.; Chen, S.; Fernandes, N.; Price, B.D. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc. Natl. Acad. Sci. USA 2005, 102, 13182–13187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingues, S.C.; Konietzko, U.; Henriques, A.G.; Rebelo, S.; Fardilha, M.; Nishitani, H.; Nitsch, R.M.; da Cruz, E.S.E.F.; da Cruz, E.S.O.A. RanBP9 modulates AICD localization and transcriptional activity via direct interaction with Tip60. J. Alzheimers Dis. 2014, 42, 1415–1433. [Google Scholar] [CrossRef] [PubMed]
- Kaidi, A.; Jackson, S.P. KAT5 tyrosine phosphorylation couples chromatin sensing to ATM signalling. Nature 2013, 498, 70–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atabakhsh, E.; Bryce, D.M.; Lefebvre, K.J.; Schild-Poulter, C. RanBPM has proapoptotic activities that regulate cell death pathways in response to DNA damage. Mol. Cancer Res. 2009, 7, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Palavicini, J.P.; Lloyd, B.N.; Hayes, C.D.; Bianchi, E.; Kang, D.E.; Dawson-Scully, K.; Lakshmana, M.K. RanBP9 Plays a Critical Role in Neonatal Brain Development in Mice. PLoS ONE 2013, 8, e66908. [Google Scholar] [CrossRef] [PubMed]
- Kunert, S.; Meyer, I.; Fleischhauer, S.; Wannack, M.; Fiedler, J.; Shivdasani, R.A.; Schulze, H. The microtubule modulator RanBP10 plays a critical role in regulation of platelet discoid shape and degranulation. Blood 2009, 114, 5532–5540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuoka, S.; Ballif, B.A.; Smogorzewska, A.; McDonald, E.R., 3rd; Hurov, K.E.; Luo, J.; Bakalarski, C.E.; Zhao, Z.; Solimini, N.; Lerenthal, Y.; et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007, 316, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Beli, P.; Lukashchuk, N.; Wagner, S.A.; Weinert, B.T.; Olsen, J.V.; Baskcomb, L.; Mann, M.; Jackson, S.P.; Choudhary, C. Proteomic investigations reveal a role for RNA processing factor THRAP3 in the DNA damage response. Mol. Cell 2012, 46, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Pines, A.; Kelstrup, C.D.; Vrouwe, M.G.; Puigvert, J.C.; Typas, D.; Misovic, B.; de Groot, A.; von Stechow, L.; van de Water, B.; Danen, E.H.; et al. Global phosphoproteome profiling reveals unanticipated networks responsive to cisplatin treatment of embryonic stem cells. Mol. Cell. Biol. 2011, 31, 4964–4977. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.E.; Boardman, A.P.; Wang, D.C.; Huttlin, E.L.; Everley, R.A.; Dephoure, N.; Zhou, C.; Koren, I.; Gygi, S.P.; Elledge, S.J. Quantitative Proteomic Atlas of Ubiquitination and Acetylation in the DNA Damage Response. Mol. Cell 2015, 59, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Oei, A.L.; Vriend, L.E.; Crezee, J.; Franken, N.A.; Krawczyk, P.M. Effects of hyperthermia on DNA repair pathways: One treatment to inhibit them all. Radiat. Oncol. 2015, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- van Oorschot, B.; Granata, G.; Di Franco, S.; Ten Cate, R.; Rodermond, H.M.; Todaro, M.; Medema, J.P.; Franken, N.A. Targeting DNA double strand break repair with hyperthermia and DNA-PKcs inhibition to enhance the effect of radiation treatment. Oncotarget 2016, 7, 65504–65513. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.; Caspari, T. When heat casts a spell on the DNA damage checkpoints. Open Biol. 2014, 4, 140008. [Google Scholar] [CrossRef] [PubMed]

| Protein Domain | Residue | AA | Target Peptide | PhosphoSitePlus | |
|---|---|---|---|---|---|
| RANBP9 (Uniprot Q96S59) | PRY | 181 | S | KFSYIGLSQNNLRVH | 1 |
| LisH | 375 | S | MIQKMVSSYLVHHGY | - | |
| CTLH | 426 | T | MGEAIETTQQLYPSL | - | |
| Post-CTLH Region | 470 | S | LGGRSPKSQDSYPVS | 2 | |
| 483 | S | VSPRPFSSPSMSPSH | 58 | ||
| 550 | S | NSINMSRSQQVNNFT | 1 | ||
| 585 | S | NGFLNGSSKHDHEME | - | ||
| 603 | S | TEMEVDSSQLRRQLC | 2 | ||
| 613 | S | RRQLCGGSQAAIERM | 2 | ||
| CRA | 631 | S | GRELQAMSEQLRRDC | - | |
| 705 | T | ALAMGQATQCLGLMA | - | ||
| RANBP10 (Uniprot Q6VN20) | PRY | 69 | S | KYNYIGLSQGNLRVH | 1 |
| LisH | 263 | S | VLQNMVSSYLVHHGY | - | |
| CTLH | 314 | T | VGEAIETTQRFYPGL | - | |
| Post-CTLH Region | 358 | S | LSSRSPKSQDSYPGS | 5 | |
| 365 | S | SQDSYPGSPSLSPRH | 160 | ||
| 377 | S | PRHGPSSSHMHNTGA | - | ||
| 386 | S | MHNTGADSPSCSNGV | 7 | ||
| 439 | S | NSTDSTKSQHHSSTS | - | ||
| 490 | S | DLQTDESSMDDRHPR | 13 | ||
| CRA | 579 | S | LNSAILESQNLPKQP | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmieri, D.; Tessari, A.; Coppola, V. Scorpins in the DNA Damage Response. Int. J. Mol. Sci. 2018, 19, 1794. https://doi.org/10.3390/ijms19061794
Palmieri D, Tessari A, Coppola V. Scorpins in the DNA Damage Response. International Journal of Molecular Sciences. 2018; 19(6):1794. https://doi.org/10.3390/ijms19061794
Chicago/Turabian StylePalmieri, Dario, Anna Tessari, and Vincenzo Coppola. 2018. "Scorpins in the DNA Damage Response" International Journal of Molecular Sciences 19, no. 6: 1794. https://doi.org/10.3390/ijms19061794





