Aspirin is Involved in the Cell Cycle Arrest, Apoptosis, Cell Migration, and Invasion of Oral Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Results
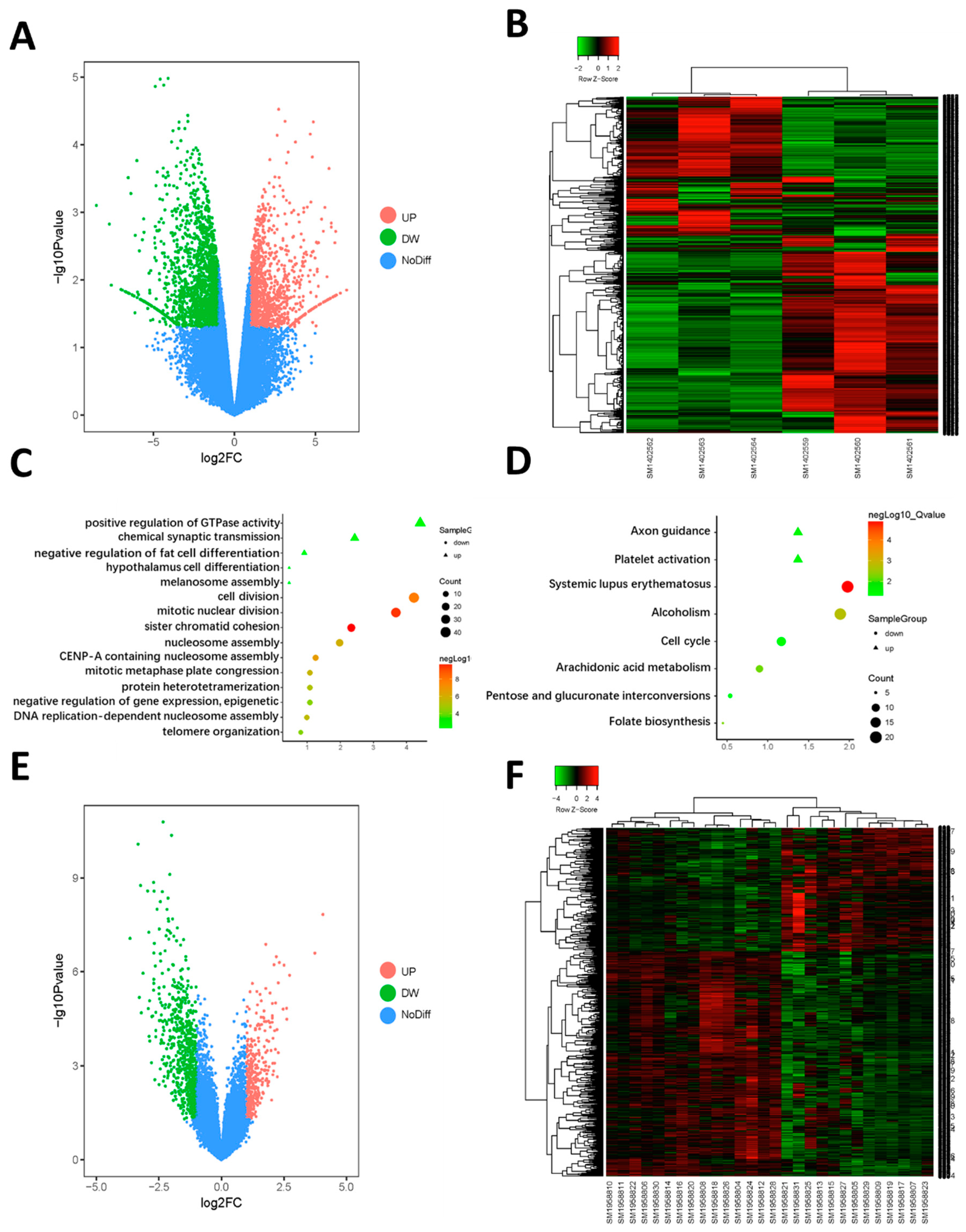
2.1. Identification of the DEGs
2.2. Function Enrichment Analysis
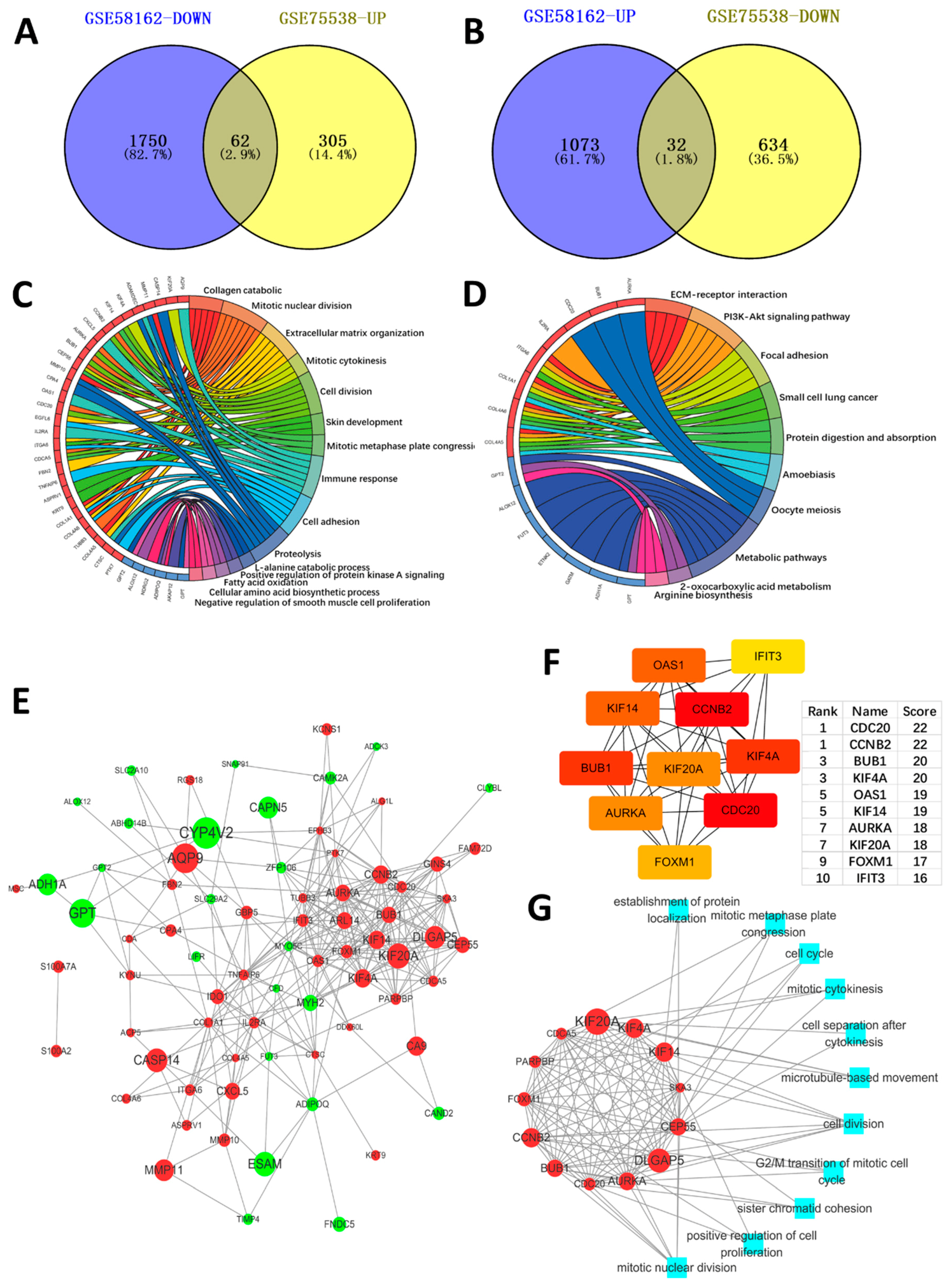
2.3. PPI Network Construction, Significant Modules Identification, and Modules Analysiss
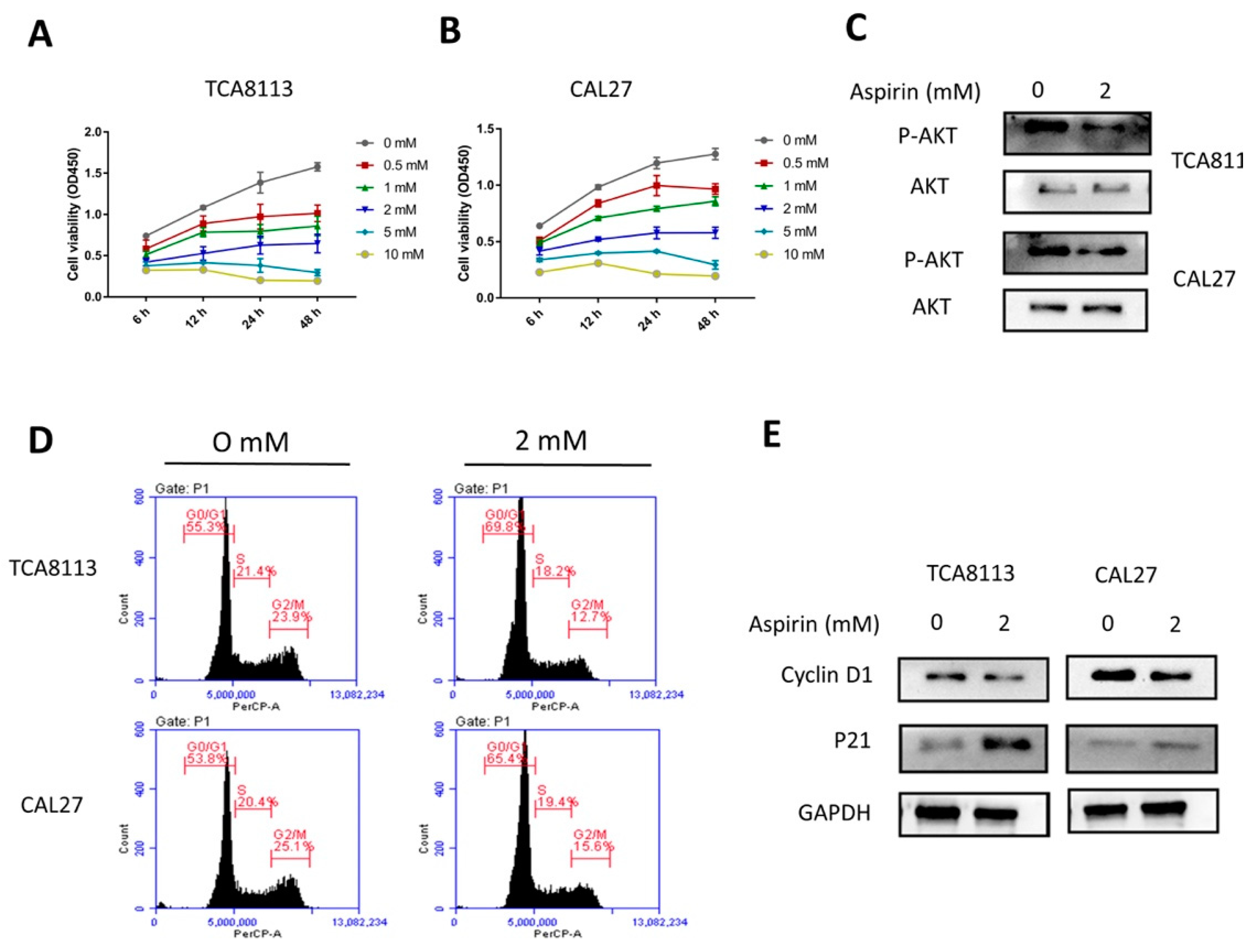
2.4. Aspirin Inhibits Cell Viability in a Dose- and Time-Dependent Manner
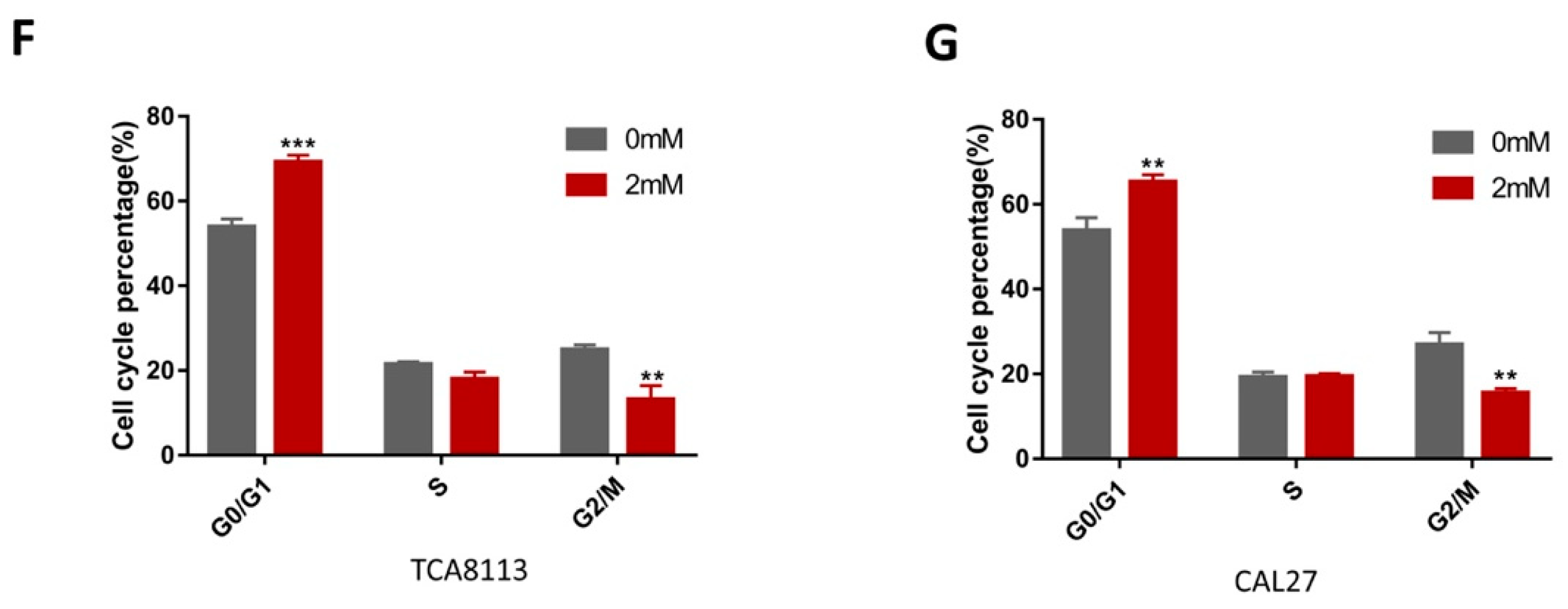
2.5. Aspirin-Induced G0/G1 Arrest
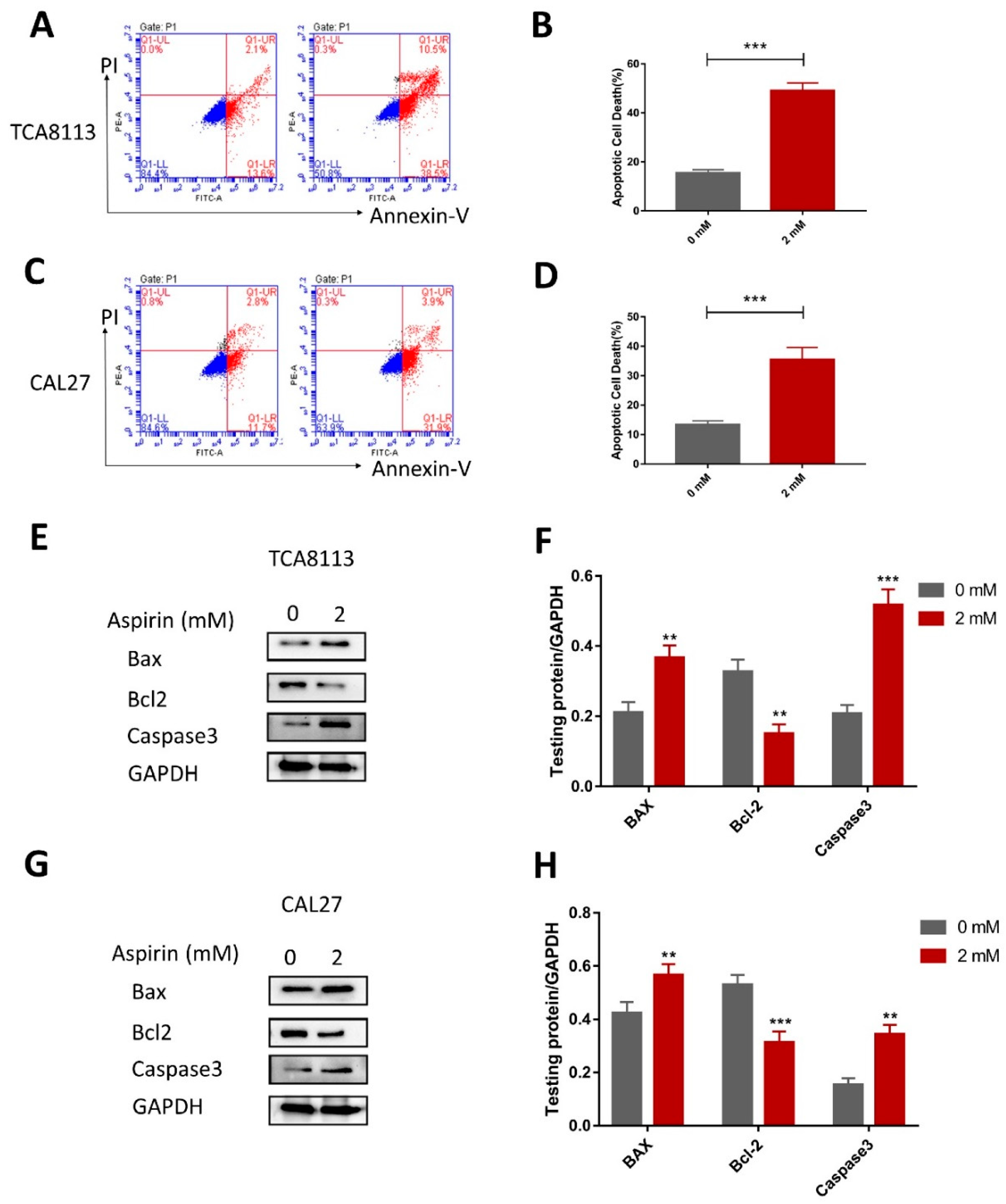
2.6. Aspirin Induces Apoptosis in TCA8113 and CAL27 Cells
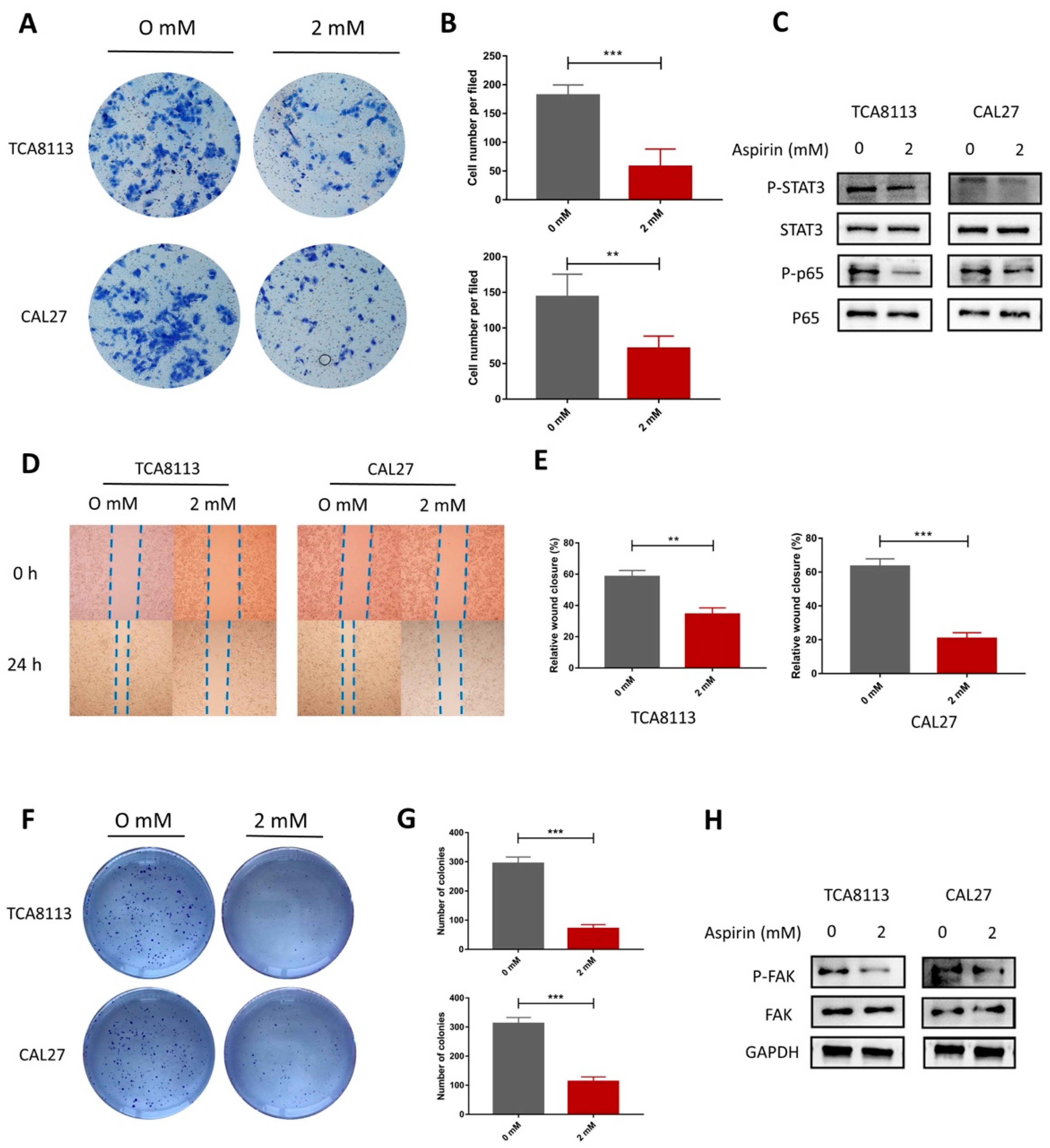
2.7. Aspirin Inhibits Migration, Invasion and Cloning Formation in TCA8113 and CAL27 Cells
3. Discussion
4. Materials and Methods
4.1. Data Processing for Identification of DEGs
4.2. Gene Ontology and Pathway Enrichment Analysis
4.3. Protein–Protein Interaction (PPI) Network Construction
4.4. Cells and Reagents
4.5. Cell Viability Assay
4.6. Invasion Assay
4.7. Scratch Assay
4.8. Clone Formation Assay
4.9. Cell Cycle Analysis
4.10. Apoptosis Flow-Cytometry Assay
4.11. Western Blot ANALYSIS
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OSCC | oral squamous cell carcinoma |
| DEGs | differentially methylated genes |
| PPI | protein–protein interaction |
| GO | gene ontology |
| ASA | Aspirin |
| COX | cyclooxygenases |
| NSAID | non-steroidal anti-inflammatory drug |
| CC | cell component |
| BP | biological process |
| MF | molecular function |
| ECM | extracellular matrix |
References
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Messadi, D.V.; Sato, K. Oral cancer chernoprevention: Current status and future direction. J. Calif. Dent. Assoc. 2016, 44, 101–111. [Google Scholar] [PubMed]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sayans, M.; Somoza-Martin, J.M.; Barros-Angueira, F.; Diz, P.G.; Rey, J.M.; Garcia-Garcia, A. Multidrug resistance in oral squamous cell carcinoma: The role of vacuolar atpases. Cancer Lett. 2010, 295, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wu, J.; Xu, W.; Nie, H.; Zhou, R.; Wang, R.; Liu, Y.; Tang, G.; Wu, J. Salvanic acid b inhibits glycolysis in oral squamous cell carcinoma via targeting PI3K/Akt/hif-1alpha signaling pathway. Cell Death Dis. 2018, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Ondrey, F.G.; Juhn, S.K.; Adams, G.L. Inhibition of head and neck tumor cell growth with arachidonic acid metabolism inhibition. Laryngoscope 1996, 106, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Epstein, J.B. Carcinogenesis and cyclooxygenase: The potential role of cox-2 inhibition in upper aerodigestive tract cancer. Oral Oncol. 2003, 39, 537–546. [Google Scholar] [CrossRef]
- Dalen, J.E. Aspirin to prevent heart attack and stroke: What’s the right dose? Am. J. Med. 2006, 119, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.C.; Anderson, L.A.; Murray, L.J.; Hughes, C.M. Non-steroidal anti-inflammatory drug and aspirin use and the risk of head and neck cancer: A systematic review. Cancer Causes Control 2011, 22, 803–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, A.T.; Giovannucci, E.L.; Meyerhardt, J.A.; Schernhammer, E.S.; Wu, K.; Fuchs, C.S. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology 2008, 134, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E.; Namboodiri, K.K.; Farrar, W.B. Nonsteroidal antiinflammatory drugs and breast cancer. Epidemiology 1996, 7, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, K.; Di Cesar, D.; Ghosn, J.; Rajan, R.; Mahmud, S.; Rahme, E. Association between nonsteroidal anti-inflammatory drugs and prostate cancer occurrence. Cancer J. 2006, 12, 130–135. [Google Scholar] [PubMed]
- Sjodahl, R. Nonsteroidal anti-inflammatory drugs and the gastrointestinal tract. Extent, mode, and dose dependence of anticancer effects. Am. J. Med. 2001, 110, 66S–69S. [Google Scholar] [CrossRef]
- Ahmadi, N.; Goldman, R.; Seillier-Moiseiwitsch, F.; Noone, A.M.; Kosti, O.; Davidson, B.J. Decreased risk of squamous cell carcinoma of the head and neck in users of nonsteroidal anti-inflammatory drugs. Int. J. Otolaryngol. 2010, 2010, 424161. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.M.; Tergaonkar, V. Nfkappab signaling in carcinogenesis and as a potential molecular target for cancer therapy. Apoptosis 2009, 14, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Parker, J.S.; Ely, K.; Carter, J.; Yi, Y.; Murphy, B.A.; Ang, K.K.; El-Naggar, A.K.; Zanation, A.M.; Cmelak, A.J.; et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-kappab signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res. 2006, 66, 8210–8218. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.J.; Yamamoto, Y.; Gaynor, R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of i(kappa)b kinase-beta. Nature 1998, 396, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Kulasingam, V.; Diamandis, E.P. Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nat. Clin. Pract. Oncol. 2008, 5, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Demokan, S.; Chuang, A.Y.; Chang, X.; Khan, T.; Smith, I.M.; Pattani, K.M.; Dasgupta, S.; Begum, S.; Khan, Z.; Liegeois, N.J.; et al. Identification of guanine nucleotide-binding protein gamma-7 as an epigenetically silenced gene in head and neck cancer by gene expression profiling. Int. J. Oncol. 2013, 42, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Kodela, R.; Chattopadhyay, M.; Goswami, S.; Gan, Z.Y.; Rao, P.P.; Nia, K.V.; Velazquez-Martinez, C.A.; Kashfi, K. Positional isomers of aspirin are equally potent in inhibiting colon cancer cell growth: Differences in mode of cyclooxygenase inhibition. J. Pharmacol. Exp. Ther. 2013, 345, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Hernandez-Ledesma, B.; de Lumen, B.O. Lunasin, a novel seed peptide, sensitizes human breast cancer mda-mb-231 cells to aspirin-arrested cell cycle and induced apoptosis. Chem. Biol. Interact. 2010, 186, 127–134. [Google Scholar] [CrossRef] [PubMed]
- De Luna-Bertos, E.; Ramos-Torrecillas, J.; Garcia-Martinez, O.; Diaz-Rodriguez, L.; Ruiz, C. Effect of aspirin on cell growth of human mg-63 osteosarcoma line. Sci. World J. 2012, 2012, 834246. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Wilson, M.; Price, J.F.; Belch, J.F.; Meade, T.W.; Mehta, Z. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet 2012, 379, 1591–1601. [Google Scholar] [CrossRef]
- Liao, D.; Zhong, L.; Duan, T.; Zhang, R.H.; Wang, X.; Wang, G.; Hu, K.; Lv, X.; Kang, T. Aspirin suppresses the growth and metastasis of osteosarcoma through the nf-kappab pathway. Clin. Cancer Res. 2015, 21, 5349–5359. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Chakraborty, G.; Baek, K.; Yoon, H.S. Aspirin-induced bcl-2 translocation and its phosphorylation in the nucleus trigger apoptosis in breast cancer cells. Exp. Mol. Med. 2013, 45, e47. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.H.; Yuan, L.Y.; Huang, R.B.; Chen, G.A. Aspirin inhibits proliferation and induces apoptosis of multiple myeloma cells through regulation of bcl-2 and bax and suppression of vegf. Eur. J. Haematol. 2014, 93, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Li, J.; Chen, J.; Zhu, L.; Wang, Y.; Sun, B.; Hua, B.; Guo, C.; Yan, Z. Aspirin inhibits the proliferation of synovium-derived mesenchymal stem cells by arresting the cell cycle in the g0/g1 phase. Am. J. Transl. Res. 2017, 9, 5056–5062. [Google Scholar] [PubMed]
- Wu, J.S.; Sheng, S.R.; Liang, X.H.; Tang, Y.L. The role of tumor microenvironment in collective tumor cell invasion. Future Oncol. 2017, 13, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R. Cell cycle-regulatory cyclins and their deregulation in oral cancer. Oral Oncol. 2013, 49, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Friedl, P. Mapping proteolytic cancer cell-extracellular matrix interfaces. Clin. Exp. Metastasis 2009, 26, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Chang, T.H.; Huang, Y.F.; Huang, H.D.; Chou, C.Y. Col11a1 promotes tumor progression and predicts poor clinical outcome in ovarian cancer. Oncogene 2014, 33, 3432–3440. [Google Scholar] [CrossRef] [PubMed]
- McLean, G.W.; Carragher, N.O.; Avizienyte, E.; Evans, J.; Brunton, V.G.; Frame, M.C. The role of focal-adhesion kinase in cancer—A new therapeutic opportunity. Nat. Rev. Cancer 2005, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ali, S.; Fotovati, A.; Shirasuna, K. Tyrosine-kinase inhibition results in EGFR clustering at focal adhesions and consequent exocytosis in uPAR down-regulated cells of Head and Neck cancers. Mol. Cancer 2008, 7, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, F.; Lee, J.T.; Navolanic, P.M.; Steelman, L.S.; Shelton, J.G.; Blalock, W.L.; Franklin, R.A.; McCubrey, J.A. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia 2003, 17, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Fresno Vara, J.A.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; Gonzalez-Baron, M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Dillon, R.L.; White, D.E.; Muller, W.J. The phosphatidyl inositol 3-kinase signaling network: Implications for human breast cancer. Oncogene 2007, 26, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhou, W.; Zhang, Y.; Liu, Y. High expression of ptgr1 promotes nsclc cell growth via positive regulation of cyclin-dependent protein kinase complex. BioMed Res. Int. 2016, 2016, 5230642. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Kuang, J.; Koomen, J.; Kobayashi, R.; Khokhar, A.R.; Siddik, Z.H. Recruitment of trimeric proliferating cell nuclear antigen by g1-phase cyclin-dependent kinases following DNA damage with platinum-based antitumour agents. Br. J. Cancer 2013, 109, 2378–2388. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, L.J. Focal adhesion kinase expression in oral cancers. Head Neck 1998, 20, 634–639. [Google Scholar] [CrossRef]
- De Vicente, J.C.; Rosado, P.; Lequerica-Fernandez, P.; Allonca, E.; Villallain, L.; Hernandez-Vallejo, G. Focal adhesion kinase overexpression: Correlation with lymph node metastasis and shorter survival in oral squamous cell carcinoma. Head Neck 2013, 35, 826–830. [Google Scholar] [CrossRef] [PubMed]
- King, K.L.; Cidlowski, J.A. Cell cycle regulation and apoptosis. Annu. Rev. Physiol. 1998, 60, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, A.; Mascitti, M.; Lo Russo, L.; Sartini, D.; Troiano, G.; Emanuelli, M.; Lo Muzio, L. Survivin-based treatment strategies for squamous cell carcinoma. Int. J. Mol. Sci. 2018, 19, 971. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Winterford, C.M.; Harmon, B.V. Apoptosis. Its significance in cancer and cancer therapy. Cancer 1994, 73, 2013–2026. [Google Scholar] [CrossRef]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Moser, R.; Xu, C.; Kao, M.; Annis, J.; Lerma, L.A.; Schaupp, C.M.; Gurley, K.E.; Jang, I.S.; Biktasova, A.; Yarbrough, W.G.; et al. Functional kinomics identifies candidate therapeutic targets in head and neck cancer. Clin. Cancer Res. 2014, 20, 4274–4288. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Locker, J.; Sahai, E.; Segall, J.E. Classifying collective cancer cell invasion. Nat. Cell. Biol. 2012, 14, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Tiwari, R.; Nauta, J.J.; van der Waal, I.; Snow, G.B. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer 1993, 71, 452–456. [Google Scholar] [CrossRef] [Green Version]
- Jefri, M.; Huang, Y.N.; Huang, W.C.; Tai, C.S.; Chen, W.L. Ykl-40 regulated epithelial-mesenchymal transition and migration/invasion enhancement in non-small cell lung cancer. BMC Cancer 2015, 15, 590. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Zhao, X.M.; Shuai, Z.F.; Li, C.Y.; Bai, Q.Y.; Yu, X.W.; Wen, Q.T. Snail promotes epithelial-mesenchymal transition and invasiveness in human ovarian cancer cells. Int. J. Clin. Exp. Med. 2015, 8, 7388–7393. [Google Scholar] [PubMed]
- Yuan, J.; Zhang, F.; Niu, R. Multiple regulation pathways and pivotal biological functions of STAT3 in cancer. Sci. Rep. 2015, 5, 17663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, G.; Loukinova, E.; Chen, Z.; Gangi, L.; Chanturita, T.I.; Liu, E.T.; Van Waes, C. Molecular profiling of transformed and metastatic murine squamous carcinoma cells by differential display and cDNA microarray reveals altered expression of multiple genes related to growth, apoptosis, angiogenesis, and the NF-κB signal pathway. Cancer Res. 2001, 61, 4797–4808. [Google Scholar] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The string database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Feng, H.; Li, Z.; Guo, J.; Li, M. Aspirin is Involved in the Cell Cycle Arrest, Apoptosis, Cell Migration, and Invasion of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2018, 19, 2029. https://doi.org/10.3390/ijms19072029
Zhang X, Feng H, Li Z, Guo J, Li M. Aspirin is Involved in the Cell Cycle Arrest, Apoptosis, Cell Migration, and Invasion of Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2018; 19(7):2029. https://doi.org/10.3390/ijms19072029
Chicago/Turabian StyleZhang, Xiaoqi, Hao Feng, Ziyu Li, Jie Guo, and Minqi Li. 2018. "Aspirin is Involved in the Cell Cycle Arrest, Apoptosis, Cell Migration, and Invasion of Oral Squamous Cell Carcinoma" International Journal of Molecular Sciences 19, no. 7: 2029. https://doi.org/10.3390/ijms19072029




