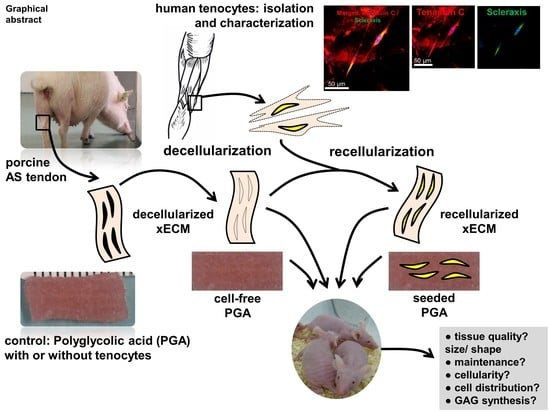
Tenogenesis of Decellularized Porcine Achilles Tendon Matrix Reseeded with Human Tenocytes in the Nude Mice Xenograft Model
Abstract
:1. Introduction
2. Results
2.1. Decellularization of the Porcine AS Tendon xECM
2.2. Recellularization of the Porcine AS Tendon xECM with Human Tenocytes In Vitro
2.3. Macroscopical Construct Quality after Implantation into the Nude Mice
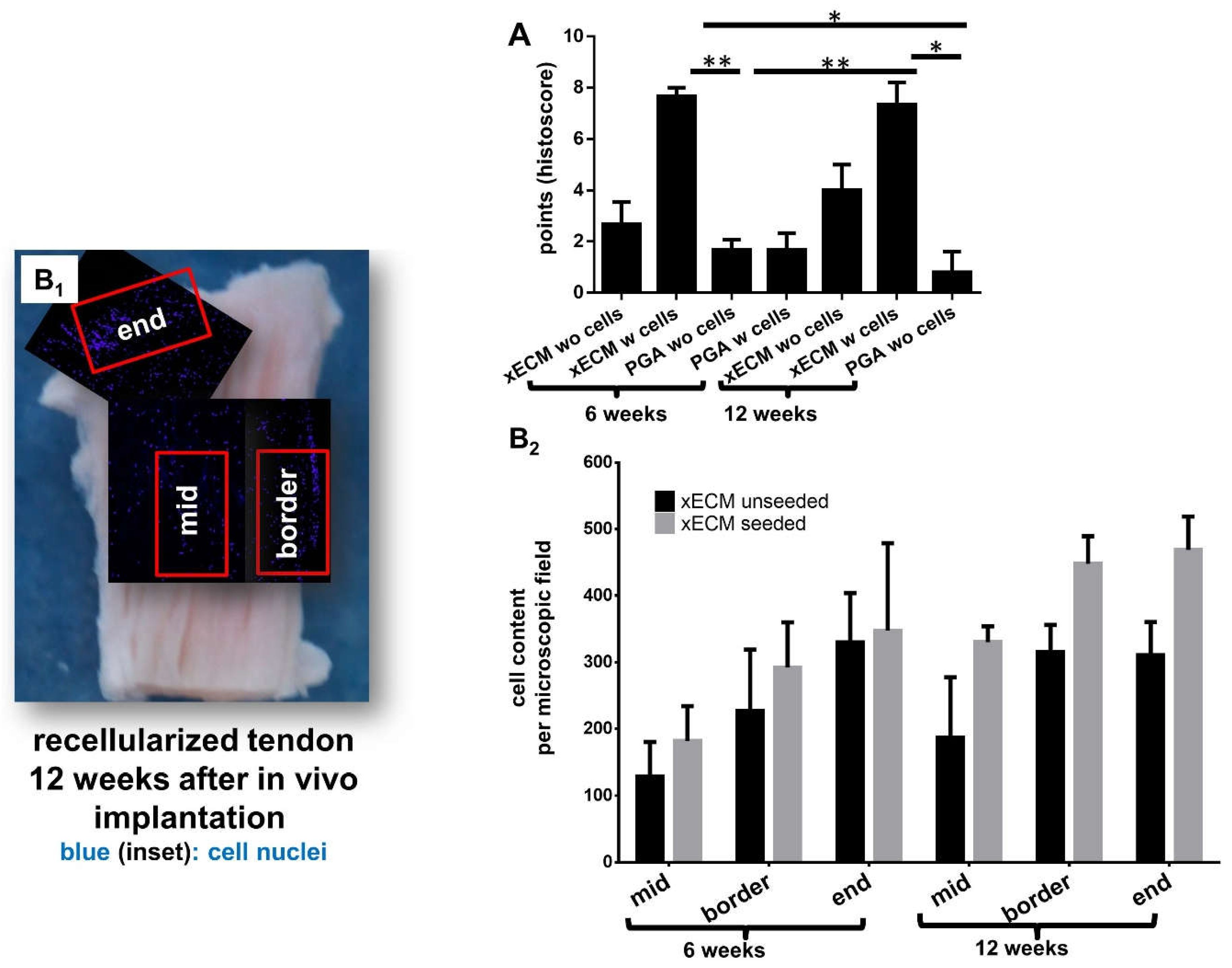
2.4. Histological Construct Quality after Implantation into the Nude Mice
2.5. Comparison of GAG Content In Vitro and In Vivo
2.7. Comparison of αSMA in Native Tendon and in Decellularized or Recellularized xECM Implanted in Nude Mice for Six and 12 Weeks
2.8. Detection of Human Cell Nuclei in Recellularized xECM and Implanted in Nude Mice for Six and 12 Weeks
3. Discussion
4. Materials and Methods
4.1. Human Tenocyte Isolation and Expansion
4.2. Porcine AS Tendon Decellularization
4.3. Recellularization of Tendon and Seeding of PGA
4.4. Vitality Testing
4.5. Histological Stainings
4.6. Nude Mice Xenograft Model
4.7. Total DNA and GAG Content Measurement
4.8. Immunolabeling of α-Smooth Muscle Actin and Anti-Human Nuclei in the Reseeded Samples
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| αSMA | α-smooth muscle actin |
| AB | Alcian blue |
| AS | Achilles |
| DAPI | 4′,6′-diamidino-2-phenylindol |
| DMMB | dimethyl methylene blue |
| ECM | extracellular matrix |
| EDTA | ethylenediaminetetraacetic acid |
| Etbr | ethidium bromide |
| FCS | fetal calf serum |
| FDA | fluorescein diacetate |
| GAG | glycosaminoglycan |
| HE | hematoxylin/eosin |
| M. | musculus |
| MSC | mesenchymal stromal cells |
| PBE | phosphate-buffered EDTA |
| PFA | paraformaldehyde |
| PGA | polyglycolic acid |
| PI | propidium iodide |
| RT | room temperature |
| SDS | sodium dodecyl sulfate |
| TBS | Tris-buffered saline |
| sGAG | sulfated glycosaminoglycans |
References
- Cheng, C.W.; Solorio, L.D.; Alsberg, E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv. 2014, 32, 462–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schandl, K.; Horvathy, D.B.; Doros, A.; Majzik, E.; Schwarz, C.M.; Csonge, L.; Abkarovits, G.; Bucsi, L.; Lacza, Z. Bone-Albumin filling decreases donor site morbidity and enhances bone formation after anterior cruciate ligament reconstruction with bone-patellar tendon-bone autografts. Int. Orthop. 2016, 40, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Cervellin, M.; de Girolamo, L.; Bait, C.; Denti, M.; Volpi, P. Autologous platelet-rich plasma gel to reduce donor-site morbidity after patellar tendon graft harvesting for anterior cruciate ligament reconstruction: A randomized, controlled clinical study. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Kartus, J.; Movin, T.; Karlsson, J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy 2001, 17, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Tsujino, J.; Ohkoshi, Y.; Tanabe, Y.; Kaneda, K. Graft site morbidity with autogenous semitendinosus and gracilis tendons. Am. J. Sports Med. 1995, 23, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Mbimba, T.; Younesi, M.; Akkus, O. Effects of substrate stiffness on the tenoinduction of human mesenchymal stem cells. Acta Biomater. 2017, 58, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Younesi, M.; Mbimba, T.; Akkus, O. Collagen Substrate Stiffness Anisotropy Affects Cellular Elongation, Nuclear Shape, and Stem Cell Fate toward Anisotropic Tissue Lineage. Adv. Healthc. Mater. 2016, 5, 2237–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishore, V.; Bullock, W.; Sun, X.; Van Dyke, W.S.; Akkus, O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials 2012, 33, 2137–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruzmetov, M.; Shah, J.J.; Geiss, D.M.; Fortuna, R.S. Decellularized versus standard cryopreserved valve allografts for right ventricular outflow tract reconstruction: A single-institution comparison. J. Thorac. Cardiovasc. Surg. 2012, 143, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.H.; Herbert, A.; Jones, G.L.; Manfield, I.W.; Fisher, J.; Ingham, E. The effects of irradiation on the biological and biomechanical properties of an acellular porcine superflexor tendon graft for cruciate ligament repair. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Herbert, A.; Berry, H.; Edwards, J.H.; Fisher, J.; Ingham, E. Decellularization and Characterization of Porcine Superflexor Tendon: A Potential Anterior Cruciate Ligament Replacement. Tissue Eng. Part A 2017, 23, 124–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smart, N.J.; Bryan, N.; Hunt, J.A.; Daniels, I.R. Porcine dermis implants in soft-tissue reconstruction: Current status. Biologics 2014, 8, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Fan, X.; Chen, P.; Shao, C.; Lu, W. Reconstruction of a tissue-engineered cornea with porcine corneal acellular matrix as the scaffold. Cells Tissues Organs 2010, 191, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Tullius, R.; Kokini, K.; Shelbourne, K.D.; Klootwyk, T.; Voytik, S.L.; Kraine, M.R.; Simmons, C. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J. Biomed. Mater. Res. 1995, 29, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.; Arnoczky, S.; Plouhar, P.; Haut, R.; Mendenhall, V.; Clarke, R.; Horvath, C. Naturally occurring extracellular matrix as a scaffold for musculoskeletal repair. Clin. Orthop. Relat. Res. 1999, S333–S343. [Google Scholar] [CrossRef]
- Dejardin, L.M.; Arnoczky, S.P.; Ewers, B.J.; Haut, R.C.; Clarke, R.B. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa. Histologic and mechanical evaluation in dogs. Am. J. Sports Med. 2001, 29, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Gogele, C.; Schwarz, S.; Ondruschka, B.; Hammer, N.; Schulze-Tanzil, G. Decellularized Iliotibial Band Recolonized with Allogenic Homotopic Fibroblasts or Bone Marrow-Derived Mesenchymal Stromal Cells. Methods Mol. Biol. 2017. [Google Scholar] [CrossRef]
- Hammer, N.; Huster, D.; Boldt, A.; Hadrich, C.; Koch, H.; Mobius, R.; Schulze-Tanzil, G.; Scheidt, H.A. A preliminary technical study on sodium dodecyl sulfate-induced changes of the nano-structural and macro-mechanical properties in human iliotibial tract specimens. J. Mech. Behav. Biomed. Mater. 2016, 61, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G.; Al-Sadi, O.; Ertel, W.; Lohan, A. Decellularized tendon extracellular matrix-a valuable approach for tendon reconstruction? Cells 2012, 1, 1010–1028. [Google Scholar] [CrossRef] [PubMed]
- Lovati, A.B.; Bottagisio, M.; Moretti, M. Decellularized and Engineered Tendons as Biological Substitutes: A Critical Review. Stem Cells Int. 2016, 2016, 7276150. [Google Scholar] [CrossRef] [PubMed]
- Lohan, A.; Stoll, C.; Albrecht, M.; Denner, A.; John, T.; Kruger, K.; Ertel, W.; Schulze-Tanzil, G. Human hamstring tenocytes survive when seeded into a decellularized porcine Achilles tendon extracellular matrix. Connect. Tissue Res. 2013, 54, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Woods, T.; Gratzer, P.F. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials 2005, 26, 7339–7349. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.H.; Korossis, S.; Howling, G.; Fisher, J.; Ingham, E. The use of ultrasonication to aid recellularization of acellular natural tissue scaffolds for use in anterior cruciate ligament reconstruction. Tissue Eng. 2007, 13, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.J.; Zhang, Y.; Chen, X.H.; Luo, J.C.; Li, X.Q.; Yang, Z.M.; Qin, T.W. Preparation and characterization of decellularized tendon slices for tendon tissue engineering. J. Biomed. Mater. Res. A 2012, 100, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.A.; Xu, Q. Biocompatibility and degradation of tendon-derived scaffolds. Regen. Biomater. 2016, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Liu, G.M.; Ning, L.J.; Zhang, Y.; Luo, J.C.; Huang, F.G.; Qin, T.W. Rotator cuff repair using a decellularized tendon slices graft: An in vivo study in a rabbit model. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Omae, H.; Sun, Y.L.; An, K.N.; Amadio, P.C.; Zhao, C. Engineered tendon with decellularized xenotendon slices and bone marrow stromal cells: An in vivo animal study. J. Tissue Eng. Regen. Med. 2012, 6, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, B.; Wang, J.H. The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials 2011, 32, 6972–6981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, T.; Fox, P.M.; Woon, C.Y.; Farnebo, S.J.; Bronstein, J.A.; Behn, A.; Pham, H.; Chang, J. Human flexor tendon tissue engineering: In vivo effects of stem cell reseeding. Plast. Reconstr. Surg. 2013, 132, 567e–576e. [Google Scholar] [CrossRef] [PubMed]
- McGoldrick, R.; Chattopadhyay, A.; Crowe, C.; Chiou, G.; Hui, K.; Farnebo, S.; Davis, C.; Le Grand, A.; Jacobs, M.; Pham, H.; et al. The Tissue-Engineered Tendon-Bone Interface: In Vitro and In Vivo Synergistic Effects of Adipose-Derived Stem Cells, Platelet-Rich Plasma, and Extracellular Matrix Hydrogel. Plast. Reconstr. Surg. 2017, 140, 1169–1184. [Google Scholar] [CrossRef] [PubMed]
- Abbah, S.A.; Spanoudes, K.; O’Brien, T.; Pandit, A.; Zeugolis, D.I. Assessment of stem cell carriers for tendon tissue engineering in pre-clinical models. Stem Cell Res. Ther. 2014, 5, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoll, C.; John, T.; Conrad, C.; Lohan, A.; Hondke, S.; Ertel, W.; Kaps, C.; Endres, M.; Sittinger, M.; Ringe, J.; et al. Healing parameters in a rabbit partial tendon defect following tenocyte/biomaterial implantation. Biomaterials 2011, 32, 4806–4815. [Google Scholar] [CrossRef] [PubMed]
- Schleifenbaum, S.; Prietzel, T.; Aust, G.; Boldt, A.; Fritsch, S.; Keil, I.; Koch, H.; Mobius, R.; Scheidt, H.A.; Wagner, M.F.; et al. Acellularization-Induced Changes in Tensile Properties Are Organ Specific—An In-Vitro Mechanical and Structural Analysis of Porcine Soft Tissues. PLoS ONE 2016, 11, e0151223. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.J.; Jiang, Y.L.; Zhang, C.H.; Zhang, Y.; Yang, J.L.; Cui, J.; Zhang, Y.J.; Yao, X.; Luo, J.C.; Qin, T.W. Fabrication and characterization of a decellularized bovine tendon sheet for tendon reconstruction. J. Biomed. Mater. Res. A 2017, 105, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Kuntz, L.A.; Foehr, P.; Kuempel, K.; Wagner, A.; Tuebel, J.; Deimling, C.V.; Burgkart, R.H. Efficient decellularization for tissue engineering of the tendon-bone interface with preservation of biomechanics. PLoS ONE 2017, 12, e0171577. [Google Scholar] [CrossRef] [PubMed]
- Rich, A.M.; Pearlstein, E.; Weissmann, G.; Hoffstein, S.T. Cartilage proteoglycans inhibit fibronectin-mediated adhesion. Nature 1981, 293, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Lohan, A.; Marzahn, U.; El Sayed, K.; Haisch, A.; Kohl, B.; Muller, R.D.; Ertel, W.; Schulze-Tanzil, G.; John, T. In vitro and in vivo neo-cartilage formation by heterotopic chondrocytes seeded on PGA scaffolds. Histochem. Cell Biol. 2011, 136, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, X.; Zhu, T.; Hu, J.J.; Song, H.X.; Shen, W.L.; Jiang, L.Y.; Heng, B.C.; Ji, J.F.; Ouyang, H.W. The effect of decellularized matrices on human tendon stem/progenitor cell differentiation and tendon repair. Acta Biomater. 2013, 9, 9317–9329. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G.; Mobasheri, A.; Clegg, P.D.; Sendzik, J.; John, T.; Shakibaei, M. Cultivation of human tenocytes in high-density culture. Histochem. Cell Biol. 2004, 122, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Stoll, C.; John, T.; Endres, M.; Rosen, C.; Kaps, C.; Kohl, B.; Sittinger, M.; Ertel, W.; Schulze-Tanzil, G. Extracellular matrix expression of human tenocytes in three-dimensional air-liquid and PLGA cultures compared with tendon tissue: Implications for tendon tissue engineering. J. Orthop. Res. 2010, 28, 1170–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeken, C.R.; White, A.K.; Bachman, S.L.; Ramshaw, B.J.; Cleveland, D.S.; Loy, T.S.; Grant, S.A. Method of preparing a decellularized porcine tendon using tributyl phosphate. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 96, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Chan, S.A.; Breuer, C.K.; Niklason, L.E. Novel utilization of serum in tissue decellularization. Tissue Eng. Part C Methods 2010, 16, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Sah, R.L.; Doong, J.Y.; Grodzinsky, A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal. Biochem. 1988, 174, 168–176. [Google Scholar] [CrossRef]
- Glough, J.V.; Alexander-Williams, J. Surgical and economic advantages of polyglycolic-acid suture material in skin closure. Lancet 1975, 1, 194–195. [Google Scholar] [PubMed]
- Massullo, J.M.; Singh, T.P.; Dunnican, W.J.; Binetti, B.R. Preliminary study of hiatal hernia repair using polyglycolic acid: Trimethylene carbonate mesh. JSLS J. Soc. Laparoendosc. Surg. 2012, 16, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Kawai, N.; Kawaguchi, T.; Suzuki, S.; Yasukawa, M.; Tojo, T.; Taniguchi, S. Low-voltage coagulation, polyglycolic acid sheets, and fibrin glue to control air leaks in lung surgery. Gen. Thorac. Cardiovasc. Surg. 2017, 65, 705–709. [Google Scholar] [CrossRef] [PubMed]







| Macroscopical Scoring | Points |
|---|---|
| color | |
| white, glossy | 2 |
| rose, rough | 1 |
| other color | 0 |
| surface structure | |
| smooth, tight, intact | 2 |
| rough | 1 |
| surface with clefts and holes | 0 |
| shape | |
| like before implantation | 2 |
| unregular, tight | 1 |
| diffuse, smooth | 0 |
| size | |
| similar like before implantation | 2 |
| shrunken or swollen | 1 |
| nearly disappeared | 0 |
| maximum value | 8 |
| Histological Scoring | |
|---|---|
| ECM | Points |
| dense, parallel organized fiber bundles | 2 |
| partly dense, partly loose and unorganized | 1 |
| loose unorganized cell-ECM composition | 0 |
| Proteoglycans (Alcian Blue Staining) | |
| normal | 2 |
| focally elevated or impaired | 1 |
| generally elevated or impaired | 0 |
| Cells | |
| Cellularity | |
| normal (5–10% of tissue volume) | 2 |
| slight or focal hypo-/hyper-cellularity | 1 |
| severe hypo-/hyper-cellularity | 0 |
| Distribution | |
| homogeneous | 1 |
| focal areas with higher/lower cell density | 0 |
| Cell Orientation | |
| in rows between ECM bundles | 2 |
| focally not orientated (10–50%) | 1 |
| >50% not orientated | 0 |
| Morphology of Cell Nuclei | |
| mostly elongated | 2 |
| mixed: 10–30% round/oval shaped | 1 |
| mostly round/oval-shaped, euchromatically or polymorph heterochromatically, tenoblasts or other extrinsic fibroblasts | 0 |
| Degeneration/Metaplasia (Alcian Blue) | |
| none | 1 |
| edema, fatty tissue, cartilage, bone or fibrinoid inclusion, cell debris deposition, fiber destruction | 0 |
| Vascularization (Within the Explant) | |
| barely detectable, very low (physiological) | 1 |
| locally or generally increased | 0 |
| Infiltrating Inflammatory Cells (Neutrophils, Macrophages, Lymphocytes, Foreign Body Cells) | |
| no | 2 |
| moderate/locally/focally | 1 |
| severe, generalized | 0 |
| maximum value | 15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohan, A.; Kohl, B.; Meier, C.; Schulze-Tanzil, G. Tenogenesis of Decellularized Porcine Achilles Tendon Matrix Reseeded with Human Tenocytes in the Nude Mice Xenograft Model. Int. J. Mol. Sci. 2018, 19, 2059. https://doi.org/10.3390/ijms19072059
Lohan A, Kohl B, Meier C, Schulze-Tanzil G. Tenogenesis of Decellularized Porcine Achilles Tendon Matrix Reseeded with Human Tenocytes in the Nude Mice Xenograft Model. International Journal of Molecular Sciences. 2018; 19(7):2059. https://doi.org/10.3390/ijms19072059
Chicago/Turabian StyleLohan, Anke, Benjamin Kohl, Carola Meier, and Gundula Schulze-Tanzil. 2018. "Tenogenesis of Decellularized Porcine Achilles Tendon Matrix Reseeded with Human Tenocytes in the Nude Mice Xenograft Model" International Journal of Molecular Sciences 19, no. 7: 2059. https://doi.org/10.3390/ijms19072059