Toughness Enhancement of PHBV/TPU/Cellulose Compounds with Reactive Additives for Compostable Injected Parts in Industrial Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Compounds and Analysis of Their Processability
2.2. Characterization
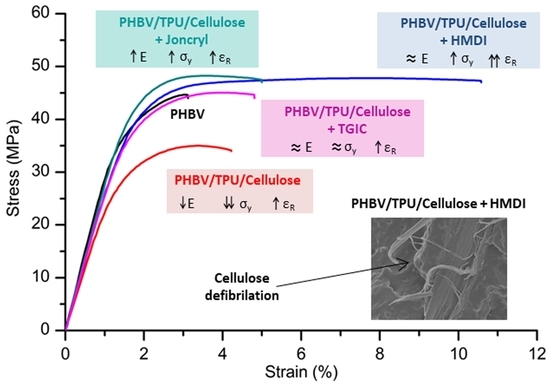
2.2.1. Mechanical Properties
2.2.2. Impact Resistance
2.2.3. Heat Deflection Temperature HDT-A
2.2.4. Biodisintegration in Composting Conditions
3. Experimental
3.1. Materials
3.2. Sample Preparation
3.3. Characterization
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Plastics Europe-Association of Plastics Manufacturers. Plastics-the Facts 2017: Analysis of European Plastics Production, Demand and Waste Data. Available online: http://www.plasticseurope.org/en/resources/publications/plastics-facts-2017 (accessed on 2 February 2018).
- Rujnić-Sokele, M.; Pilipović, A. Challenges and opportunities of biodegradable plastics: A mini review. Waste Manag. Res. 2017, 35, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.; Fernandes, B.; Covas, J.A.; Vicente, A.A.; Hilliou, L. Film blowing of PHBV blends and PHBV-based multilayers for the production of biodegradable packages. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Albuquerque, P.B.S.; Malafaia, C.B. Perspectives on the production, structural characteristics and potential applications of bioplastics derived from polyhydroxyalkanoates. Int. J. Biol. Macromol. 2018, 107, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, J.; Chen, G.Q. Polyhydroxyalkanoates, challenges and opportunities. Curr. Opin. Biotechnol. 2014, 30, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Pilla, S. Handbook of Bioplastics and Biocomposites Engineering Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 9780470626078. [Google Scholar]
- Peelman, N.; Ragaert, P.; Ragaert, K.; De Meulenaer, B.; Devlieghere, F.; Cardon, L. Heat resistance of new biobased polymeric materials, focusing on starch, cellulose, PLA, and PHA. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef] [Green Version]
- Keskin, G.; Kızıl, G.; Bechelany, M.; Pochat-Bohatier, C.; Öner, M. Potential of polyhydroxyalkanoate (PHA) polymers family as substitutes of petroleum based polymers for packaging applications and solutions brought by their composites to form barrier materials. Pure Appl. Chem. 2017, 89, 1841–1848. [Google Scholar] [CrossRef] [Green Version]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef] [Green Version]
- Väisänen, T.; Haapala, A.; Lappalainen, R.; Tomppo, L. Utilization of agricultural and forest industry waste and residues in natural fiber-polymer composites: A review. Waste Manag. 2016, 54, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Jost, V.; Miesbauer, O. Effect of different biopolymers and polymers on the mechanical and permeation properties of extruded PHBV cast films. J. Appl. Polym. Sci. 2018, 135, 46153. [Google Scholar] [CrossRef]
- Zhang, K.; Misra, M.; Mohanty, A.K. Toughened sustainable green composites from poly(3-hydroxybutyrate-co-3-hydroxyvalerate) based ternary blends and miscanthus biofiber. ACS Sustain. Chem. Eng. 2014, 2, 2345–2354. [Google Scholar] [CrossRef]
- Chikh, A.; Benhamida, A.; Kaci, M.; Pillin, I.; Bruzaud, S. Synergistic effect of compatibilizer and sepiolite on the morphology of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(butylene succinate) blends. Polym. Test. 2016, 53, 19–28. [Google Scholar] [CrossRef]
- Ma, P.; Hristova-Bogaerds, D.G.; Lemstra, P.J.; Zhang, Y.; Wang, S. Toughening of PHBV/PBS and PHB/PBS Blends via In situ Compatibilization Using Dicumyl Peroxide as a Free-Radical Grafting Initiator. Macromol. Mater. Eng. 2012, 297, 402–410. [Google Scholar] [CrossRef]
- El-Taweel, S.H.; Khater, M. Mechanical and Thermal Behavior of Blends of Poly(hydroxybutyrate-co-hydroxyvalerate) with Ethylene Vinyl Acetate Copolymer. J. Macromol. Sci. Part. B 2015, 54, 1225–1232. [Google Scholar] [CrossRef]
- Adams, B.; Abdelwahab, M.; Misra, M.; Mohanty, A.K. Injection-Molded Bioblends from Lignin and Biodegradable Polymers: Processing and Performance Evaluation. J. Polym. Environ. 2017, 1–14. [Google Scholar] [CrossRef]
- Wang, S.; Chen, W.; Xiang, H.; Yang, J.; Zhou, Z.; Zhu, M. Modification and Potential Application of Short-Chain-Length Polyhydroxyalkanoate (SCL-PHA). Polymers 2016, 8, 273. [Google Scholar] [CrossRef]
- González-Ausejo, J.; Sánchez-Safont, E.; Cabedo, L.; Gamez-Perez, J. Toughness Enhancement of Commercial Poly (Hydroxybutyrate-co-Valerate) (PHBV) by Blending with a Thermoplastic Polyurethane (TPU). J. Multiscale Model. 2016, 7, 1640008. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Abad, A.; González-Ausejo, J.; Lagarón, J.M.; Cabedo, L. Biodegradable poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/thermoplastic polyurethane blends with improved mechanical and barrier performance. Polym. Degrad. Stab. 2016, 132, 52–61. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Mohanty, A.K.; Drzal, L.T.; Pourboghrat, F.; Misra, M. Renewable resource-based green composites from recycled cellulose fiber and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastic. Biomacromolecules 2006, 7, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Biocomposites of different lignocellulosic wastes for sustainable food packaging applications. Compos. Part. B Eng. 2018, 145, 215–225. [Google Scholar] [CrossRef]
- Satyanarayana, K.G.; Arizaga, G.G.C.; Wypych, F. Biodegradable composites based on lignocellulosic fibers—An overview. Prog. Polym. Sci. 2009, 34, 982–1021. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Rosa, M.D.F.; Cioffi, M.O.H.; Benini, K.C.C.D.C.; Milanese, A.C.; Voorwald, H.J.C.; Mulinari, D.R. Vegetal fibers in polymeric composites: A review. Polímeros 2015, 25, 9–22. [Google Scholar] [CrossRef]
- Wei, L.; McDonald, A. A Review on Grafting of Biofibers for Biocomposites. Materials 2016, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Pandey, J.K.; Mohanty, A.K. Biocomposites: Design and Mechanical Performance; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9781782423942. [Google Scholar]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable compatibilized polymer blends for packaging applications: A literature review. J. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef]
- Stenstad, P.; Andresen, M.; Tanem, B.S.; Stenius, P. Chemical surface modifications of microfibrillated cellulose. Cellulose 2008, 15, 35–45. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, F.; Qian, J.; Huang, J.; Wolcott, M.; Liu, L.; Zhang, J. Reinforcing and Toughening Effects of Bamboo Pulp Fiber on Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Fiber Composites. Ind. Eng. Chem. Res. 2010, 49, 572–577. [Google Scholar] [CrossRef]
- Zarrinbakhsh, N.; Mohanty, A.K.; Misra, M. Improving the interfacial adhesion in a new renewable resource-based biocomposites from biofuel coproduct and biodegradable plastic. J. Mater. Sci. 2013, 48, 6025–6038. [Google Scholar] [CrossRef]
- Anderson, S.; Zhang, J.; Wolcott, M.P. Effect of Interfacial Modifiers on Mechanical and Physical Properties of the PHB Composite with High Wood Flour Content. J. Polym. Environ. 2013, 21, 631–639. [Google Scholar] [CrossRef]
- González-Ausejo, J.; Sánchez-Safont, E.; Lagarón, J.M.; Balart, R.; Cabedo, L.; Gámez-Pérez, J. Compatibilization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)–poly(lactic acid) blends with diisocyanates. J. Appl. Polym. Sci. 2017, 134, 1–11. [Google Scholar] [CrossRef]
- Dogan, S.K.; Reyes, E.A.; Rastogi, S.; Ozkoc, G. Reactive compatibilization of PLA/TPU blends with a diisocyanate. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Dogan, S.K.; Boyacioglu, S.; Kodal, M.; Gokce, O.; Ozkoc, G. Thermally induced shape memory behavior, enzymatic degradation and biocompatibility of PLA/TPU blends: “Effects of compatibilization”. J. Mech. Behav. Biomed. Mater. 2017, 71, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Wu, H.; Qiu, F.; Wang, X. Interface Bond Improvement of Sisal Fibre Reinforced Polylactide Composites with Added Epoxy Oligomer. Materials 2018, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Nanthananon, P.; Seadan, M.; Pivsa-Art, S.; Hiroyuki, H.; Suttiruengwong, S. Biodegradable polyesters reinforced with eucalyptus fiber: Effect of reactive agents. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2017; p. 70012. [Google Scholar]
- Tang, W.; Wang, H.; Tang, J.; Yuan, H. Polyoxymethylene/thermoplastic polyurethane blends compatibilized with multifunctional chain extender. J. Appl. Polym. Sci. 2013, 127, 3033–3039. [Google Scholar] [CrossRef]
- Hao, M.; Wu, H. Effect of in situ reactive interfacial compatibilization on structure and properties of polylactide/sisal fiber biocomposites. Polym. Compos. 2018, 39, E174–E187. [Google Scholar] [CrossRef]
- Ferrero, B.; Fombuena, V.; Fenollar, O.; Boronat, T.; Balart, R. Development of natural fiber-reinforced plastics (NFRP) based on biobased polyethylene and waste fibers from Posidonia oceanica seaweed. Polym. Compos. 2015, 36, 1378–1385. [Google Scholar] [CrossRef]
- Hameed, N.; Guo, Q.; Tay, F.H.; Kazarian, S.G. Blends of cellulose and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) prepared from the ionic liquid 1-butyl-3-methylimidazolium chloride. Carbohydr. Polym. 2011, 86, 94–104. [Google Scholar] [CrossRef]
- Mechanical and biodegradation performance of short natural fibre polyhydroxybutyrate composites. Polym. Test. 2013, 32, 1603–1611.
- Wu, S. Phase structure and adhesion in polymer blends: A criterion for rubber toughening. Polymer 1985, 26, 1855–1863. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; Arrillaga, A.; Anakabe, J.; Gamez-Perez, J.; Cabedo, L. PHBV/TPU/Cellulose compounds for compostable injection molded parts with improved thermal and mechanical performance. J. Appl. Polym. Sci. submitted.
- Seggiani, M.; Cinelli, P.; Mallegni, N.; Balestri, E.; Puccini, M.; Vitolo, S.; Lardicci, C.; Lazzeri, A. New Bio-Composites Based on Polyhydroxyalkanoates and Posidonia oceanica Fibres for Applications in a Marine Environment. Materials 2017, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Yatigala, N.S.; Bajwa, D.S.; Bajwa, S.G. Compatibilization improves physico-mechanical properties of biodegradable biobased polymer composites. Compos. Part. A Appl. Sci. Manuf. 2018, 107, 315–325. [Google Scholar] [CrossRef]
- Wang, S.; Xiang, H.; Wang, R.; Peng, C.; Zhou, Z.; Zhu, M. Morphology and properties of renewable poly(3-hydroxybutyrate-co-3-hydroxyvalerate) blends with thermoplastic polyurethane. Polym. Eng. Sci. 2014, 54, 1113–1119. [Google Scholar] [CrossRef]
- Margolina, A.; Wu, S. Percolation model for brittle-tough transition in nylon/rubber blends. Polymer 1988, 29, 2170–2173. [Google Scholar] [CrossRef]
- Nagarajan, V.; Misra, M.; Mohanty, A.K. New engineered biocomposites from poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/poly(butylene adipate-co-terephthalate) (PBAT) blends and switchgrass: Fabrication and performance evaluation. Ind. Crops Prod. 2013, 42, 461–468. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Reactive compatibilization and performance evaluation of miscanthus biofiber reinforced poly(hydroxybutyrate-co-hydroxyvalerate) biocomposites. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Rossa, L.V.; Scienza, L.C.; Zattera, A.J. Effect of curauá fiber content on the properties of poly(hydroxybutyrate-co-valerate) composites. Polym. Compos. 2013, 34, 450–456. [Google Scholar] [CrossRef]
- Buchdahl, R. Mechanical properties of polymers and composites—Vols. I and II, Lawrence, E. Nielsen, Marcel Dekker, Inc.; New York, 1974, Vol. I 255 pp. Vol. II 301 pp. Vol. I $24.50, Vol. II $28.75. J. Polym. Sci. Polym. Lett. Ed. 1975, 13, 120–121. [Google Scholar] [CrossRef]
- González-Ausejo, J.; Sanchez-Safont, E.; Lagaron, J.M.; Olsson, R.T.; Gamez-Perez, J.; Cabedo, L. Assessing the thermoformability of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(acid lactic) blends compatibilized with diisocyanates. Polym. Test. 2017, 62, 235–245. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; González-Ausejo, J.; Gámez-Pérez, J.; Lagarón, J.M.; Cabedo, L. Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate)/Purified Cellulose Fiber Composites by Melt Blending: Characterization and Degradation in Composting Conditions. J. Renew. Mater. 2016, 4, 123–132. [Google Scholar] [CrossRef]
- ISO. Determinación del Grado de Desintegración de Materiales Plásticos Bajo Condiciones de Compostaje Simuladas en un Laboratorio; UNE-EN ISO UNE-EN ISO 20200; ISO: Geneva, Switzerland, 2006. [Google Scholar]










| Sample | TPU | Cellulose | HMDI | Joncryl® | TGIC |
|---|---|---|---|---|---|
| (phr) * | |||||
| Neat PHBV | - | - | - | - | - |
| PHBV/30T/10C ** | 30 | 10 | - | - | - |
| PHBV/30T/10C-0.3HMDI | 30 | 10 | 0.3 | - | - |
| PHBV/30T/10C-0.5HMDI | 30 | 10 | 0.5 | - | - |
| PHBV/30T/10C-1HMDI | 30 | 10 | 1 | - | - |
| PHBV/30T/10C-0.3Joncryl | 30 | 10 | - | 0.3 | - |
| PHBV/30T/10C-0.5Joncryl | 30 | 10 | - | 0.5 | - |
| PHBV/30T/10C-1Joncryl | 30 | 10 | - | 1 | - |
| PHBV/30T/10C-0.3TGIC | 30 | 10 | - | - | 0.3 |
| PHBV/30T/10C-0.5TGIC | 30 | 10 | - | - | 0.5 |
| PHBV/30T/10C-1TGIC | 30 | 10 | - | - | 1 |
| PHBV/30T/30C *** | 30 | 30 | - | - | - |
| PHBV/30T/30C-0.3HMDI | 30 | 30 | 0.3 | - | - |
| PHBV/30T/30C-0.5HMDI | 30 | 30 | 0.5 | - | - |
| PHBV/30T/30C-1HMDI | 30 | 30 | 1 | - | - |
| PHBV/30T/30C-0.3Joncryl | 30 | 30 | - | 0.3 | - |
| PHBV/30T/30C-0.5Joncryl | 30 | 30 | - | 0.5 | - |
| PHBV/30T/30C-1Joncryl | 30 | 30 | - | 1 | - |
| PHBV/30T/30C-0.3TGIC | 30 | 30 | - | - | 0.3 |
| PHBV/30T/30C-0.5TGIC | 30 | 30 | - | - | 0.5 |
| PHBV/30T/30C-1TGIC | 30 | 30 | - | - | 1 |
| (phr) | PHBV/30T/10C | PHBV/30T/30C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| d10 (μm) | d50 (μm) | d90 (μm) | d (μm) | T (μm) | d10 (μm) | d50 (μm) | d90 (μm) | d (μm) | T (μm) | |
| 0 | 0.17 | 0.34 | 0.79 | 0.42 | 0.13 | 0.17 | 0.35 | 0.77 | 0.42 | 0.13 |
| 0.3 HMDI | 0.14 | 0.31 | 0.79 | 0.40 | 0.12 | 0.09 | 0.20 | 0.57 | 0.27 | 0.08 |
| 0.5 HMDI | 0.11 | 0.21 | 0.51 | 0.26 | 0.08 | 0.09 | 0.18 | 0.76 | 0.31 | 0.10 |
| 1 HMDI | 0.11 | 0.26 | 1.01 | 0.43 | 0.13 | 0.09 | 0.21 | 0.77 | 0.33 | 0.10 |
| 0.3 Joncryl | 0.09 | 0.20 | 0.52 | 0.26 | 0.08 | 0.07 | 0.14 | 0.38 | 0.19 | 0.06 |
| 0.5 Joncryl | 0.09 | 0.17 | 0.43 | 0.22 | 0.07 | 0.08 | 0.14 | 0.48 | 0.21 | 0.07 |
| 1 Joncryl | 0.09 | 0.16 | 0.39 | 0.20 | 0.06 | 0.07 | 0.14 | 0.36 | 0.18 | 0.06 |
| 0.3 TGIC | 0.10 | 0.21 | 0.57 | 0.28 | 0.09 | 0.07 | 0.14 | 0.43 | 0.20 | 0.06 |
| 0.5 TGIC | 0.09 | 0.21 | 0.52 | 0.26 | 0.08 | 0.08 | 0.14 | 0.39 | 0.20 | 0.06 |
| 1 TGIC | 0.08 | 0.17 | 0.54 | 0.25 | 0.08 | 0.09 | 0.17 | 0.42 | 0.21 | 0.07 |
| Sample | HDT-A (°C) | |||
|---|---|---|---|---|
| PHBV/TPU/Cellulose | 0.3 phr | 0.5phr | 1 phr | |
| PHBV | 108 ± 1 | |||
| PHBV/30T/10C | 94 ± 3 | |||
| PHBV/30T/10C + HMDI | 93 ± 1 | 98 ± 3 | 95 ± 4 | |
| PHBV/30T/10C + Joncryl | 95 ± 1 | 98 ± 3 | 97 ± 1 | |
| PHBV/30T/10C + TGIC | 90 ± 3 | 90 ± 1 | 90 ± 3 | |
| PHBV/30T/30C | 96 ± 1 | |||
| PHBV/30T/30C + HMDI | 97 ± 2 | 99 ± 2 | 100 ± 1 | |
| PHBV/30T/30C + Joncryl | 98 ± 4 | 97 ± 3 | 94 ± 1 | |
| PHBV/30T/30C + TGIC | 104 ± 3 | 99 ± 3 | 99 ± 1 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Safont, E.L.; Arrillaga, A.; Anakabe, J.; Cabedo, L.; Gamez-Perez, J. Toughness Enhancement of PHBV/TPU/Cellulose Compounds with Reactive Additives for Compostable Injected Parts in Industrial Applications. Int. J. Mol. Sci. 2018, 19, 2102. https://doi.org/10.3390/ijms19072102
Sánchez-Safont EL, Arrillaga A, Anakabe J, Cabedo L, Gamez-Perez J. Toughness Enhancement of PHBV/TPU/Cellulose Compounds with Reactive Additives for Compostable Injected Parts in Industrial Applications. International Journal of Molecular Sciences. 2018; 19(7):2102. https://doi.org/10.3390/ijms19072102
Chicago/Turabian StyleSánchez-Safont, Estefanía Lidón, Alex Arrillaga, Jon Anakabe, Luis Cabedo, and Jose Gamez-Perez. 2018. "Toughness Enhancement of PHBV/TPU/Cellulose Compounds with Reactive Additives for Compostable Injected Parts in Industrial Applications" International Journal of Molecular Sciences 19, no. 7: 2102. https://doi.org/10.3390/ijms19072102