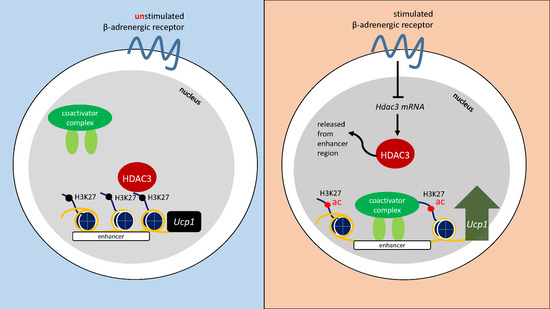
β-adrenergic Receptor Stimulation Revealed a Novel Regulatory Pathway via Suppressing Histone Deacetylase 3 to Induce Uncoupling Protein 1 Expression in Mice Beige Adipocyte
Abstract
:1. Introduction
2. Results
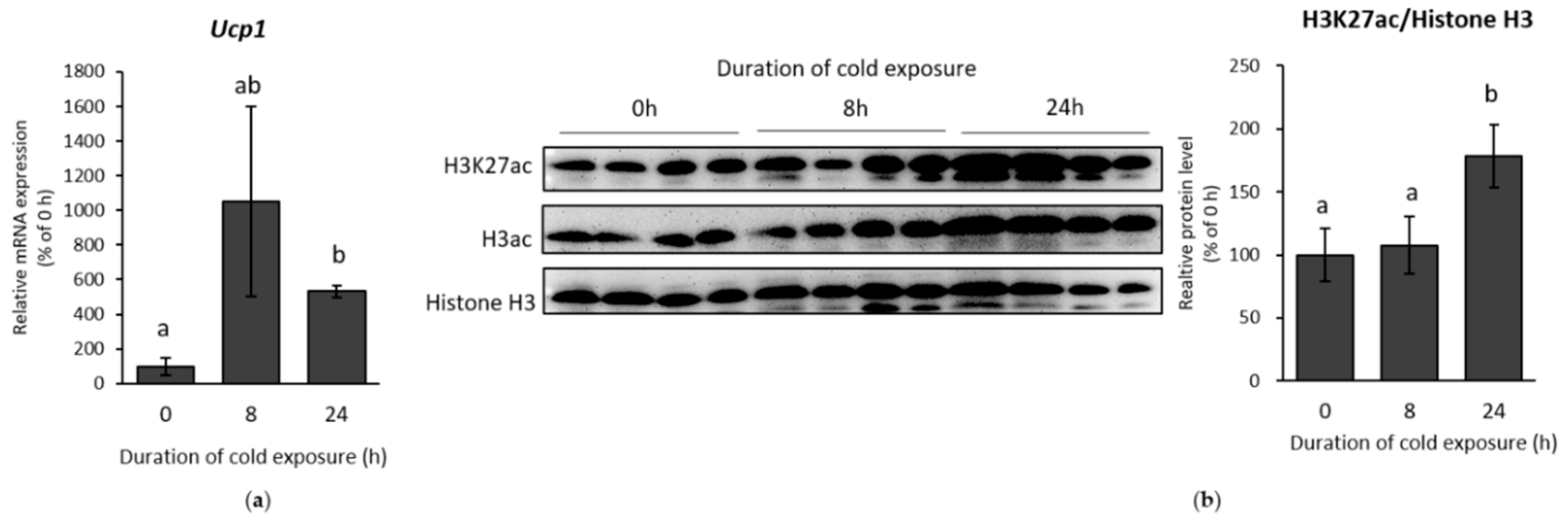
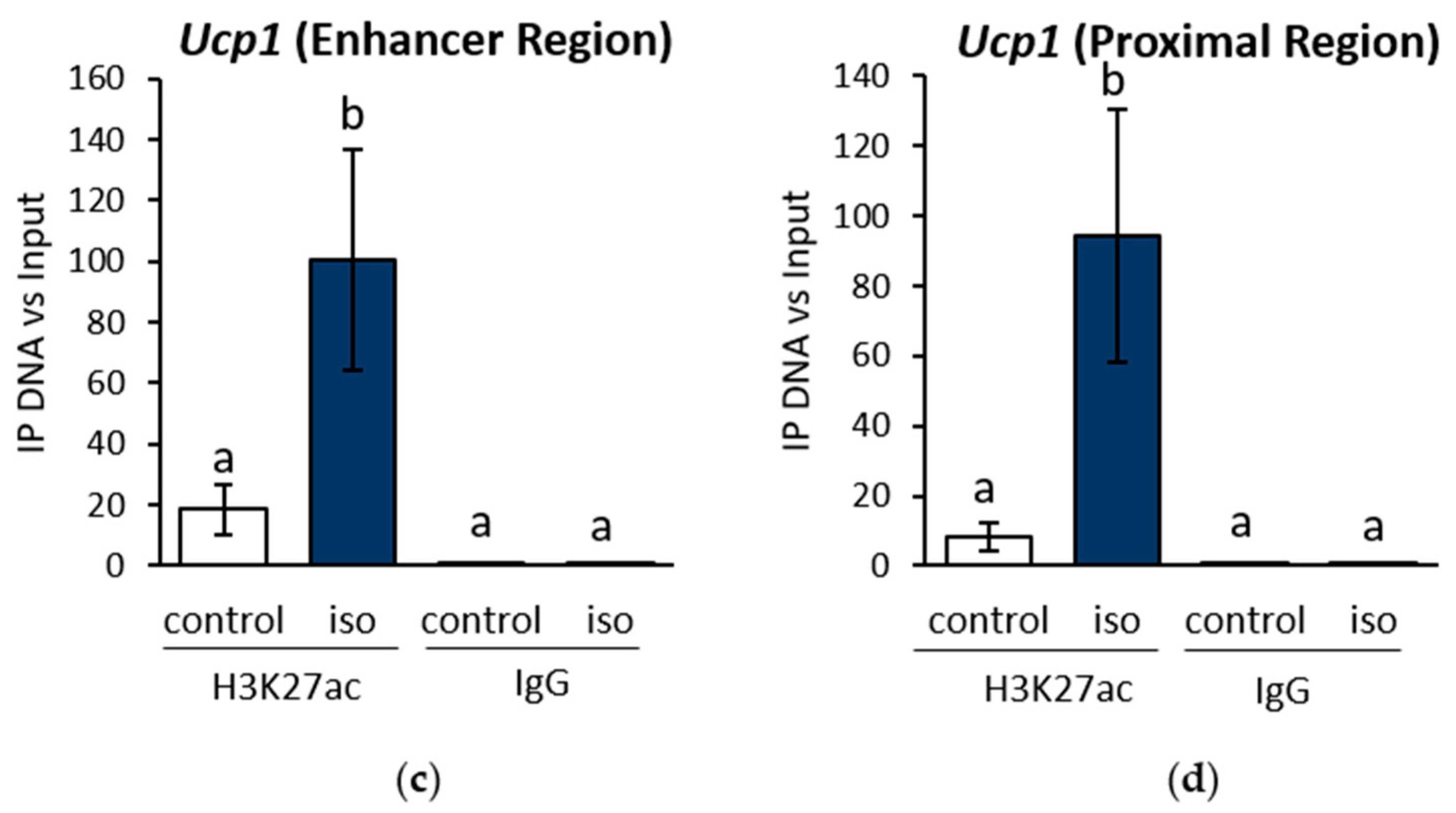
2.1. β-AR Stimulation-Induced Acetylation of Histone 3 Lysine 27 Favouring Open Chromatin Structure in the Ucp1 Promoter Region
2.2. β-AR-Stimulated Ucp1 Transcriptional Activation Is Associated with Inhibition of Class I But not Class II HDAC in IWAT Cell
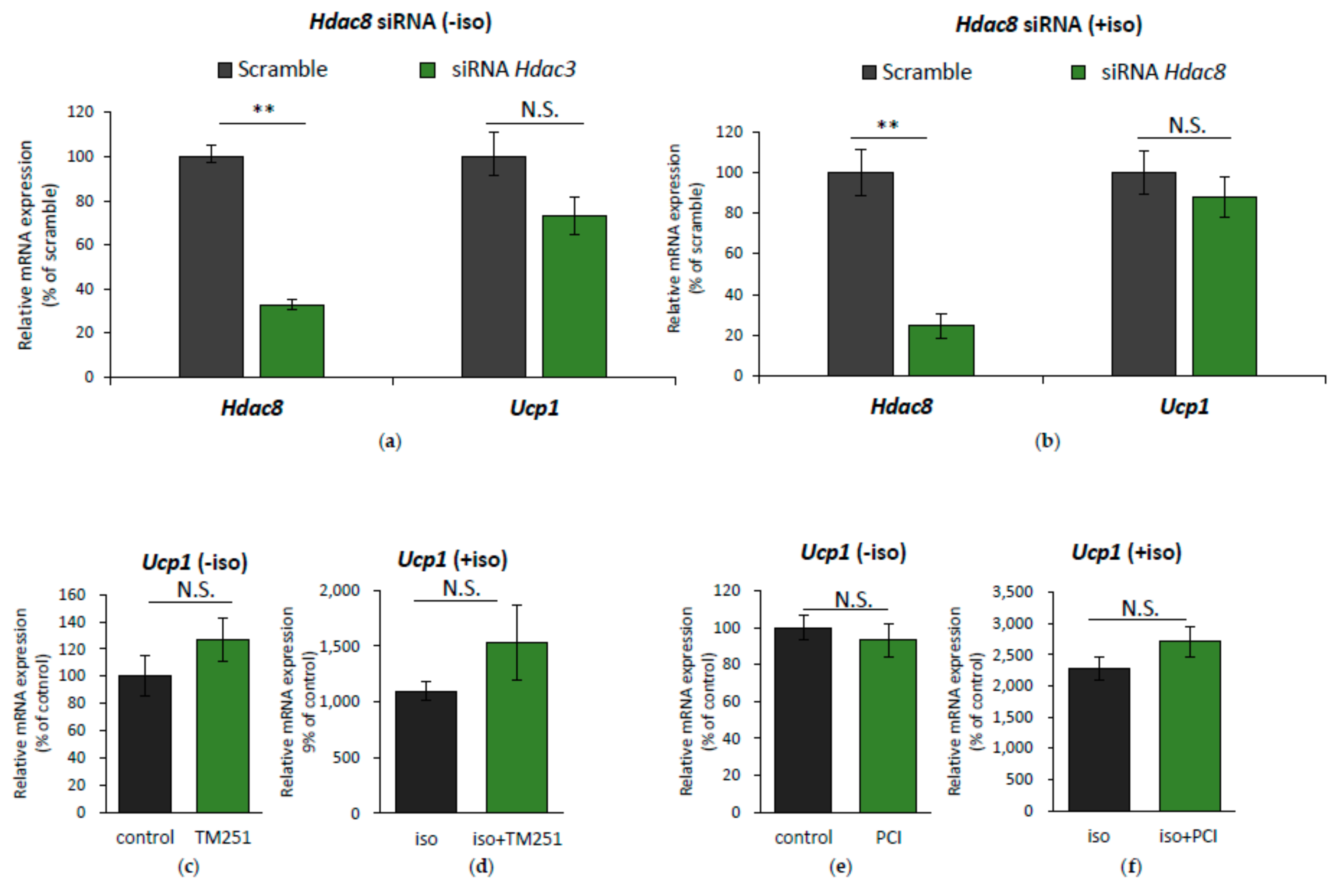
2.3. HDAC8 Might not Be Involved in Ucp1 Regulation in IWAT Cell
2.4. HDAC3 Inhibition Plays a Major Role in Ucp1 Transcriptional Activation During β-AR Stimulation in IWAT Cell
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Experiments
4.3. Cell Culture
4.4. RNA Preparation and Quantification of Gene Expression
4.5. Immunoblotting
4.6. HDAC Activity Assay
4.7. Chromatin Immunoprecipitation (ChIP) Assay
4.8. Small Interfering RNA (siRNA) Transfection
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| β-AR | beta-adrenergic receptor |
| BAT | brown adipose tissue |
| ChIP | chromatin immunoprecipitation |
| CRE | cAMP response element |
| H3ac | histone 3 acetylation |
| H3K27 | histone 3 lysine 27 |
| HDAC | histone deacetylase |
| HDI | HDAC inhibitor |
| IWAT | inguinal white adipose tissue |
| PGC1α | peroxisome proliferator-activated receptor gamma coactivator 1 alpha |
| PKA | protein kinase A |
| PPAR | peroxisome proliferator-activated receptor |
| siRNA | small interfering RNA |
| UCP1 | uncoupling protein 1 |
| WAT | white adipose tissue |
References
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Yehuda-Shnaidman, E.; Wang, H. Positive and negative control of Ucp1 gene transcription and the role of b-adrenergic signaling networks. Int. J. Obes. 2010, 34, S28–S33. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Kalinovich, A.V.; de Jong, J.M.A.; Cannon, B.; Nedergaard, J. UCP1 in adipose tissues: Two steps to full browning. Biochimie 2017, 134, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Coulter, A.; Rim, J.S.; Koza, R.A.; Kozak, L.P. Transcriptional Synergy and the Regulation of Ucp1 during Brown Adipcytes Induction in White Fat Depots. Mol. Cell. Biol. 2005, 25, 8311–8322. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, E.P.; Balasubramanian, P.; Lee, Y.-H.; Weng, C.; Kershaw, E.E.; Granneman, J.G. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic β3-adrenergic receptor activation. J. Lipid Res. 2014, 55, 2276–2286. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.L.; Formosa, M.F.; Van Every, B.; Bertovic, D.; Eikelis, N.; Lambert, G.W.; Kalff, V.; Duffy, S.J.; Cherk, M.H.; Kingwell, B.A. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia 2013, 56, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Redman, L.M.; de Jonge, L.; Fang, X.; Gamlin, B.; Recker, D.; Greenway, F.L.; Smith, S.R.; Ravussin, E. Lack of an Effect of a Novel β3-Adrenoceptor Agonist, TAK-677, on Energy Metabolism in Obese Individuals: A Double-Blind, Placebo-Controlled Randomized Study. J. Clin. Endocrinol. Metab. 2007, 92, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.R.; Gennemark, P.; O’Mahony, G.; Bartesaghi, S. Unlock the thermogenic potential of adipose tissue: Pharmacological modulation and implications for treatment of diabetes and obesity. Front. Endocrinol. (Lausanne) 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Shahbazian, M.D.; Grunstein, M. Functions of Site-Specific Histone Acetylation and Deacetylation. Annu. Rev. Biochem. 2007, 76, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Galmozzi, A.; Mitro, N.; Ferrari, A.; Gers, E.; Gilardi, F.; Godio, C.; Cermenati, G.; Gualerzi, A.; Donetti, E.; Rotili, D.; et al. Inhibition of class i histone deacetylases unveils a mitochondrial signature and enhances oxidative metabolism in skeletal muscle and adipose tissue. Diabetes 2013, 62, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, R.; Cui, X.; Zha, L.; Yu, L.; Shi, H.; Xue, B. Histone deacetylase 1 (HDAC1) negatively regulates thermogenic program in brown adipocytes via coordinated regulation of histone H3 lysine 27 (H3K27) deacetylation and methylation. J. Biol. Chem. 2016, 291, 4523–4536. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Longo, R.; Fiorino, E.; Silva, R.; Mitro, N.; Cermenati, G.; Gilardi, F.; Desvergne, B.; Andolfo, A.; Magagnotti, C.; et al. HDAC3 is a molecular brake of the metabolic switch supporting white adipose tissue browning. Nat. Commun. 2017, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Heintzman, N.D.; Hon, G.C.; Hawkins, R.D.; Kheradpour, P.; Stark, A.; Harp, L.F.; Ye, Z.; Lee, L.K.; Stuart, R.K.; Ching, C.W.; et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 2009, 459, 108–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creyghton, M.P.; Cheng, A.W.; Welstead, G.G.; Kooistra, T.; Carey, B.W.; Steine, E.J.; Hanna, J.; Lodato, M.A.; Frampton, G.M.; Sharp, P.A.; et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA 2010, 107, 21931–21936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calo, E.; Wysocka, J. Modification of enhancer chromatin: What, how and why? Mol. Cell 2013, 49, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Bertrand, P. Inside HDACs with more selective HDAC inhibitors. Eur. J. Med. Chem. 2016, 121, 451–483. [Google Scholar] [CrossRef] [PubMed]
- De Ruijter, A.J.M.; van Gennip, A.H.; Caron, H.N.; Kemp, S.; van Kuilenburg, A.B.P. Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem. J. 2003, 370, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Groop, L. Epigenetics: A molecular link between environmental factors and type 2 diabetes. Diabetes 2009, 58, 2718–2725. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Hong, J.; Li, H.; Hu, Y.; Jia, L.; Cai, D.; Zhao, R. Butyrate stimulates adipose lipolysis and mitochondrial OXPHOS through histone hyperacetylation-associated AR3β activation in high-fat diet-induced obese mice. Exp. Physiol. 2016, 2, 273–281. [Google Scholar] [CrossRef]
- Chriett, S.; Zerzaihi, O.; Vidal, H.; Pirola, L. The histone deacetylase inhibitor sodium butyrate improves insulin signalling in palmitate-induced insulin resistance in L6 rat muscle cells through epigenetically-mediated up-regulation of Irs1. Mol. Cell. Endocrinol. 2017, 439, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Rumberger, J.M.; Arch, J.R.S.; Green, A. Butyrate and other short-chain fatty acids increase the rate of lipolysis in 3T3-L1 adipocytes. PeerJ 2014, 2, e611. [Google Scholar] [CrossRef] [PubMed]
- Ye, J. Improving insulin sensitivity with HDAC inhibitor. Diabetes 2013, 62, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Fiorino, E.; Longo, R.; Barilla, S.; Mitro, N.; Cermenati, G.; Giudici, M.; Caruso, D.; Mai, A.; Guerrini, U.; et al. Attenuation of diet-induced obesity and induction of white fat browning with a chemical inhibitor of histone deacetylases. Int. J. Obes. (Lond.) 2016, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Chiocca, S.; Segré, C.V. Regulating the regulators: The post-translational code of class i HDAC1 and HDAC2. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. Metabolic reprogramming by class I and II histone deacetylases. Trends Endocrinol. Metab. 2013, 24, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Rezai-Zadeh, N.; Seto, E. Negative regulation of histone deacetylase 8 activity by cyclic AMP-dependent protein kinase A. Mol. Cell. Biol. 2004, 24, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Heinzel, T.; Krämer, O.H. Histone deacetylases: Salesmen and customers in the post-translational modification market. Biol. Cell 2009, 101, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Henagan, T.M.; Stefanska, B.; Fang, Z.; Navard, A.M.; Ye, J.; Lenard, N.R.; Devarshi, P.P. Sodium butyrate epigenetically modulates high-fat diet-induced skeletal muscle mitochondrial adaptation, obesity and insulin resistance through nucleosome positioning. Br. J. Pharmacol. 2015, 172, 2782–2798. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Jia, Y.; Pan, S.; Jia, L.; Li, H.; Han, Z.; Cai, D.; Zhao, R. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget 2016, 7, 56071–56082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leus, N.G.J.; Van Der Wouden, P.E.; Van Den Bosch, T.; Hooghiemstra, W.T.R.; Ourailidou, M.E.; Kistemaker, L.E.M.; Bischoff, R.; Gosens, R.; Haisma, H.J.; Dekker, F.J. HDAC 3-selective inhibitor RGFP966 demonstrates anti-inflammatory properties in RAW 264.7 macrophages and mouse precision-cut lung slices by attenuating NF-κB p65 transcriptional activity. Biochem. Pharmacol. 2016, 108, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, J.P.; Sriramula, S.; Pariaut, R.; Guggilam, A.; Mariappan, N.; Elks, C.M.; Francis, J. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension 2010, 56, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Xiao, X.; Li, N.; Li, Y. Histone deacetylases inhibitors (HDACis) as novel therapeutic application in various clinical diseases. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 72, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, F.; Iglesias, R.; Giralt, M. PPARs in the control of uncoupling proteins gene expression. PPAR Res. 2007, 2007. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delcuve, G.P.; Khan, D.H.; Davie, J.R. Roles of histone deacetylases in epigenetic regulation: Emerging paradigms from studies with inhibitors. Clin. Epigenet. 2012, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Emmett, M.J.; Lim, H.; Jager, J.; Richter, H.J.; Adlanmerini, M.; Peed, L.C.; Briggs, E.R.; Steger, D.J.; Ma, T.; Sims, C.A.; et al. Histone deacetylase 3 prepares brown adipose tissue for acute thermogenic challenge. Nature 2017, 546, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Medvedev, A.V.; Daniel, K.W.; Collins, S. beta-adrenergic activation of p38 MAP kinase in adipocytes: CAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 map kinase. J. Biol. Chem. 2001, 276, 27077–27082. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Liu, Q.; Salter, A.M.; Lomax, M.A. Synergism between cAMP and PPAR γ signalling in the initiation of UCP1 gene expression in HIB1B brown adipocytes. PPAR Res. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine acetylation targets protein complexes and co-regulated major cellular functions. Science 2009, 325, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, N.A.; Ann Pitcairn, C.; Fierke, C.A. HDAC8 substrates: Histones and beyond. Biopolymers 2013, 99, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.M.; Yao, Y.L. Beyond histone and deacetylase: An overview of cytoplasmic histone deacetylases and their nonhistone substrates. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Oehme, I.; Witt, O.; Oliveira, G.; Sippl, W.; Romier, C.; Pierce, R.J.; Jung, M. HDAC8: A multifaceted target for therapeutic interventions. Trends Pharmacol. Sci. 2015, 36, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Mottamal, M.; Zheng, S.; Huang, T.L.; Wang, G. Histone Deacetylase Inhibitors in Clinical Studies as Templates for New Anticancer Agents. Molecules 2015, 20, 3898–3941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarti, A.; Melesina, J.; Kolbinger, F.R.; Oehme, I.; Senger, J.; Witt, O.; Sippl, W.; Jung, M. Targeting histone deacetylase 8 as a therapeutic approach to cancer and neurodegenerative diseases. Future Med. Chem. 2016, 8, 1609–1634. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Taliyan, R. Histone deacetylase inhibitors: Future therapeutics for insulin resistance and type 2 diabetes. Pharmacol. Res. 2016, 113, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.P.; Dahllof, M.; Lundh, M.; Rasmussen, D.N.; Nielsen, M.D.; Billestrup, N.; Grunnet, L.G.; Poulsen, T.M. Histone Deacetylase (HDAC) Inhibition as a Novel Treatment as Novel Treatment for Ddiabetes Mellitus. Mol. Med. 2011, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Leus, N.G.J.; Zwinderman, M.R.H.; Dekker, F.J. Histone deacetylase 3 (HDAC 3) as emerging drug target in NF-κB-mediated inflammation. Curr. Opin. Chem. Biol. 2016, 33, 160–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arguelles, A.O.; Meruvu, S.; Bowman, J.D. Are epigenetic drugs for diabetes and obesity at our door step? Drug Discov. Today 2016, 21, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, C.; Prabu, P.; Balakumar, M.; Lenin, R.; Prabhu, D.; Anjana, R.M.; Mohan, V.; Balasubramanyam, M. Augmentation of histone deacetylase 3 (HDAC3) epigenetic signature at the interface of proinflammation and insulin resistance in patients with type 2 diabetes. Clin. Epigenet. 2016, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.; Uemura, T.; Mizoguchi, N.; Lee, J.Y.; Taketani, K.; Nakano, Y.; Hoshino, S.; Tsuge, N.; Narukami, T.; Yu, R.; et al. Diosgenin attenuates inflammatory changes in the interaction between adipocytes and macrophages. Mol. Nutr. Food Res. 2010, 54, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Hirai, S.; Goto, T.; Kuroyanagi, K.; Lee, J.Y.; Uemura, T.; Ezaki, Y.; Takahashi, N.; Kawada, T. Dehydroabietic acid, a phytochemical, acts as ligand for PPARs in macrophages and adipocytes to regulate inflammation. Biochem. Biophys. Res. Commun. 2008, 369, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.E.; Li, Y.; Nishimura, A.; Jheng, H.F.; Yuliana, A.; Kitano-Ohue, R.; Nomura, W.; Takahashi, N.; Kim, C.S.; Yu, R.; et al. Synthesized enone fatty acids resembling metabolites from gut microbiota suppress macrophage-mediated inflammation in adipocytes. Mol. Nutr. Food Res. 2017, 61, 1–13. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward | Reverse |
|---|---|---|
| Ucp1 | 5′-CAAAGTCCGCCTTCAGATCC-3′ | 5′-AGCCGGCTGAGATCTTGTTT-3′ |
| Adrb3 | 5′-GCACCTTAGGTCTCATTATGG-3′ | 5′-GCGAAAGTCCGGGCTGCGGCAGTA-3′ |
| Pgc1α | 5′-CCCTGCCATTGTTAAGACC-3′ | 5′-TGCTGCTGTTCCTGTTTTC-3′ |
| Pparα | 5′-TCGCGTACGGCAATGGCTTTT-3′ | 5′-CTTTCATCCCCAAGCGTAGGAGG-3′ |
| Pparγ | 5′-GGAGATCTCCAGTGATATCGACCA-3′ | 5′-ACGGCTTCTACGGATCGAAAACT-3′ |
| Hdac1 | 5′-CCCATGAAGCCTCACCGAAT-3′ | 5′-CAAACACCGGACAGTCCTCA-3′ |
| Hdac2 | 5′-CTGTCTCGCTGGTGTTTTGC-3′ | 5′-GTCATTTCTTCAGCAGTGGCT-3′ |
| Hdac3 | 5′-ATGTGCCGCTTCCATTCTGA-3′ | 5′-TGGCATGATGTAGACCACCG-3′ |
| Hdac8 | 5′-ACTTGACCGGGGTCATCCTA-3′ | 5′-AACCGCTTGCATCAACACAC-3′ |
| Hdac6 | 5′-CAGCAGGATTTGCCCACCTA-3′ | 5′-TCTCCAGGACCTCCCAGAAG-3′ |
| Hdac7 | 5′-TGGGGGATCCTGAGTACCTG-3′ | 5′-GTCCACCCTCTAAGGCCAAC-3′ |
| Hdac9 | 5′-CCCACCACACATCACTGGAT-3′ | 5′-TCCATCCTTCCGCCTGAGTA-3′ |
| 36B4 | 5′-TCCTTCTTCCAGGCTTTGGG-3′ | 5′-GACACCCTCCAGAAAGCGAG-3′ |
| Gene | Forward | Reverse |
|---|---|---|
| Ucp1 enhancer | 5′-CTCCTCTACAGCGTCACAGAGG-3′ | 5-AGTCTGAGGAAAGGGTTGA-3′ |
| Ucp1 proximal | 5′-CCCACTAGCAGCTCTTTGGA-3′ | 5-CTGTGGAGCAGCTCAAAGGT-3′ |
| Gene | Sequence |
|---|---|
| Hdac3 | CAGCAUGACAUGUGCCGCUUCCAUU |
| Hdac8 | GACGGAAAUUUGACCGUAUUCUCUA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuliana, A.; Jheng, H.-F.; Kawarasaki, S.; Nomura, W.; Takahashi, H.; Ara, T.; Kawada, T.; Goto, T. β-adrenergic Receptor Stimulation Revealed a Novel Regulatory Pathway via Suppressing Histone Deacetylase 3 to Induce Uncoupling Protein 1 Expression in Mice Beige Adipocyte. Int. J. Mol. Sci. 2018, 19, 2436. https://doi.org/10.3390/ijms19082436
Yuliana A, Jheng H-F, Kawarasaki S, Nomura W, Takahashi H, Ara T, Kawada T, Goto T. β-adrenergic Receptor Stimulation Revealed a Novel Regulatory Pathway via Suppressing Histone Deacetylase 3 to Induce Uncoupling Protein 1 Expression in Mice Beige Adipocyte. International Journal of Molecular Sciences. 2018; 19(8):2436. https://doi.org/10.3390/ijms19082436
Chicago/Turabian StyleYuliana, Ana, Huei-Fen Jheng, Satoko Kawarasaki, Wataru Nomura, Haruya Takahashi, Takeshi Ara, Teruo Kawada, and Tsuyoshi Goto. 2018. "β-adrenergic Receptor Stimulation Revealed a Novel Regulatory Pathway via Suppressing Histone Deacetylase 3 to Induce Uncoupling Protein 1 Expression in Mice Beige Adipocyte" International Journal of Molecular Sciences 19, no. 8: 2436. https://doi.org/10.3390/ijms19082436