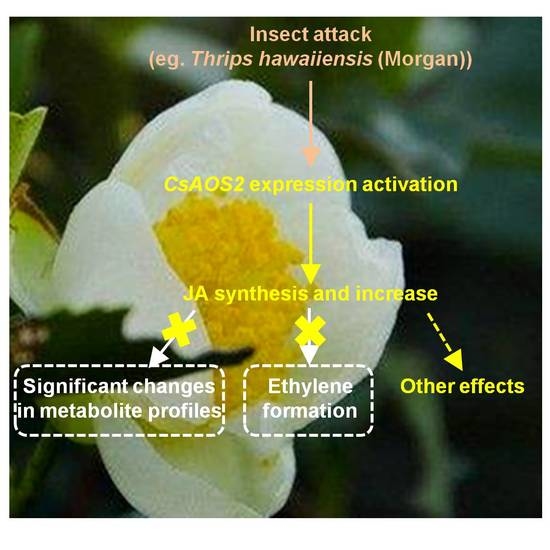
Functional Characterization of An Allene Oxide Synthase Involved in Biosynthesis of Jasmonic Acid and Its Influence on Metabolite Profiles and Ethylene Formation in Tea (Camellia sinensis) Flowers
Abstract
:1. Introduction
2. Results
2.1. Insect Attack-Upregulated CsAOS2 Functions in JA Synthesis in Tea Flowers
2.1.1. Phylogenetic Analysis of CsAOSs and Their Subcellular Localization
2.1.2. Transient Expression Analysis in N. benthamiana Plants Confirmed That CsAOS2 Functioned in JA Synthesis
2.2. In Contrast to Tea Leaves, the Metabolite Profiles of Tea Flowers Were Not Significantly Affected by JA Treatment
2.3. JA Treatment Did Not Influence Ethylene Formation in Tea Flowers
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Extraction and Analysis of Volatile Metabolites
4.3. Extraction and Analysis of Nonvolatile Metabolites
4.4. Gene Cloning
4.5. Subcellular Location Analysis of CsAOSs
4.6. Analysis of the Activities of CsAOS2 in N. benthamiana Overexpression Lines
4.7. Transcript Expression Analyses of Genes in Ethylene Formation in Tea Flowers
4.8. Collection and Analysis of Ethylene in Tea Flowers
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AOS | Allene oxide synthase |
| JA | Jasmonic acid |
| LA | Linoleic acid |
| LOX | Lipoxygenases |
| AOC | Allene oxide cyclase |
| AdoMet | S-adenosyl-l-methionine |
| SAM | S-adenosyl-l-methionine synthetase |
| ACC | 1-Aminocyclopropane-1-carboxylic acid |
| ACS | ACC synthase |
| ACO | ACC oxidase |
| EIN | Ethylene-insensitive |
| 13-HPOT | 13(S)-hydroxy linolenic acid |
| GC-MS | Gas chromatography-mass spectrometry |
| UPLC-QTOF-MS | Ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry |
References
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Dong, F.; Fu, X.M.; Watanabe, N.; Su, X.G.; Yang, Z.Y. Recent advances in the emission and functions of plant vegetative volatiles. Molecules 2016, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; MithÖfer, A.; Boland, W. Before gene expression: Early events in plant-insect interaction. Trends Plant Sci. 2007, 12, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Negre, F.; Kish, C.M.; Boatright, J.; Underwood, B.; Shibuya, K.; Wagner, C.; Clark, D.G.; Dudareva, N. Regulation of methylbenzoate emission after pollination in snapdragon and petunia flowers. Plant Cell 2003, 15, 2992–3006. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Hettenhausen, C.; Meldau, S.; Baldwin, I.T. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell 2007, 19, 1096–1122. [Google Scholar] [CrossRef] [PubMed]
- Neiland, M.R.M.; Wilcock, C.C. Fruit set, nectar reward, and rarity in the Orchidaceae. Am. J. Bot. 1998, 85, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Zhou, Y.; Zeng, L.T.; Dong, F.; Tu, Y.Y.; Yang, Z.Y. Occurrence of functional molecules in the flowers of tea (Camellia sinensis) plants: Evidence for a second resource. Molecules 2018, 23, 790. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Wu, S.S.; Lin, J.K. Determination of tea polyphenols and caffeine in tea flowers (Camellia sinensis) and their hydroxyl radical scavenging and nitric oxide suppressing effects. J. Agric. Food Chem. 2003, 51, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Tu, Y.Y.; Baldermann, S.; Dong, F.; Xu, Y.; Watanabe, N. Isolation and identification of compounds from the ethanolic extract of flowers of the tea (Camellia sinensis) plant and their contribution to the antioxidant capacity. LWT Food Sci. Technol. 2009, 42, 1439–1443. [Google Scholar] [CrossRef]
- Wang, L.; Xu, R.; Hu, B.; Li, W.; Sun, Y.; Tu, Y.; Zeng, X. Analysis of free amino acids in Chinese teas and flower of tea plant by high performance liquid chromatography combined with solid-phase extraction. Food Chem. 2010, 123, 1259–1266. [Google Scholar] [CrossRef]
- Dong, F.; Yang, Z.Y.; Baldermann, S.; Kajitani, Y.; Ota, S.; Kasuga, H.; Imazeki, Y.; Ohnishi, T.; Watanabe, N. Characterization of L-phenylalanine metabolism to acetophenone and 1-phenylethanol in the flowers of Camellia sinensis using stable isotope labeling. J. Plant Physiol. 2012, 169, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Dong, F.; Baldermann, S.; Murata, A.; Tu, Y.Y.; Asai, T.; Watanabe, N. Isolation and identification of spermidine derivatives in flowers of tea (Camellia sinensis) plants and their distributions in floral organs. J. Sci. Food Agric. 2012, 92, 2128–2132. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dong, F.; Kunimasa, A.; Zhang, Y.; Cheng, S.; Lu, J.; Zhang, L.; Murata, A.; Mayer, F.; Fleischmann, P.; et al. Occurrence of glycosidically conjugated 1-phenylethanol and its hydrolase β-primeverosidase in tea (Camellia sinensis) flowers. J. Agric. Food Chem. 2014, 62, 8042–8050. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhou, Y.; Zeng, L.T.; Peng, Q.Y.; Zhang, L.; Su, X.G.; Watanabe, N.; Yang, Z.Y. Elucidation of differential accumulation of 1-phenylethanol in flowers and leaves of tea (Camellia sinensis) plants. Molecules 2016, 21, E1106. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Nakamura, S.; Morikawa, T.; Muraoka, O.; Yoshikawa, M. New biofunctional effects of the flower buds of Camellia sinensis and its bioactive acylated oleanane-type triterpene oligoglycosides. J. Nat. Med. 2016, 70, 689–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Zeng, L.T.; Liao, Y.Y.; Dong, F.; Peng, Q.Y.; Li, J.L.; Tang, J.C.; Watanabe, N.; Yang, Z.Y. Insect (Thrips hawaiiensis (Morgan)) change the stereochemical configuration of 1-phenylethanol emitted from tea (Camellia sinensis) flowers. RSC Adv. 2017, 7, 32336–32343. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Q.Y.; Zeng, L.T.; Tang, J.C.; Li, J.L.; Dong, F.; Yang, Z.Y. Study of the biochemical formation pathway of aroma compound 1-phenylethanol in tea (Camellia sinensis) flowers and other plants. Food Chem. 2018, 258, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Yang, Z.Y.; Baldermann, S.; Sato, Y.; Asai, T.; Watanabe, N. Herbivore-induced volatiles from tea (Camellia sinensis) plants and their involvement in intraplant communication and changes in endogenous nonvolatile metabolites. J. Agric. Food Chem. 2011, 59, 13131–13135. [Google Scholar] [CrossRef] [PubMed]
- Han, B.Y.; Chen, Z.M. Composition of the volatiles from intact and mechanically pierced tea aphid-tea shoot complexes and their attraction to natural enemies of the tea aphid. J. Agric. Food Chem. 2002, 50, 2571–2575. [Google Scholar] [CrossRef] [PubMed]
- Ishiwari, H.; Suzuki, T.; Maeda, T. Essential compounds in herbivore-induced plant volatiles that attract the predatory mite Neoseiulus womersleyi. J. Chem. Ecol. 2007, 33, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.T.; Liao, Y.Y.; Li, J.L.; Zhou, Y.; Tang, J.C.; Dong, F.; Yang, Z.Y. α-Farnesene and ocimene induce metabolite changes by volatile signaling in neighboring tea (Camellia sinensis) plants. Plant Sci. 2017, 264, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kongrit, D.; Jisaka, M.; Iwanaga, C.; Yokomichi, H.; Katsube, T.; Nishimura, K.; Nagaya, T.; Yokota, K. Molecular cloning and functional expression of soybean allene oxide synthases. Biosci. Biotechnol. Biochem. 2007, 71, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Schilmiller, A.L.; McCaig, B.C.; Howe, G.A. Identification of a jasmonate-regulated allene oxide synthase that metabolizes 9-hydroperoxides of linoleic and linolenic acids. J. Biol. Chem. 2002, 277, 46051–46058. [Google Scholar] [CrossRef] [PubMed]
- Sivasankar, S.; Sheldrick, B.; Rothstein, S.J. Expression of allene oxide synthase determines defense gene activation in tomato. Plant Physiol. 2000, 122, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Farmaki, T.; Sanmartín, M.; Jiménez, P.; Paneque, M.; Sanz, C.; Vancanneyt, G.; León, J.; Sánchez-Serrano, J.J. Differential distribution of the lipoxygenase pathway enzymes within potato chloroplasts. J. Exp. Bot. 2007, 58, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Song, W.C.; Brash, A.R. Purification of an allene oxide synthase and identification of the enzyme as a cytochrome P-450. Science 1991, 253, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Song, W.C.; Funk, C.D.; Brash, A.R. Molecular cloning of an allene oxide synthase: A cytochrome P-450 specialized for metabolism of fatty acid hydroperoxides. Proc. Natl. Acad. Sci. USA 1993, 90, 8519–8523. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.G.; Ellis, C.; Devoto, A. The jasmonate signal pathway. Plant Cell 2002, 14, S1531–S1564. [Google Scholar] [CrossRef]
- Farmer, E.E.; Ryan, C.A. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 1992, 4, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A. Jasmonates as signals in the wound response. J. Plant Growth Regul. 2004, 23, 223–237. [Google Scholar] [CrossRef]
- Keinänen, M.; Oldham, N.J.; Baldwin, I.T. Rapid HPLC screening of Jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. J. Agric. Food Chem. 2001, 49, 3553–3558. [Google Scholar] [CrossRef] [PubMed]
- Porat, R.; Borochov, A.; Halevy, A.H. Enhancement of petunia and dendrobium flower senescence by jasmonic acid methyl ester is via the promotion of ethylene production. Plant Growth Regul. 1993, 13, 297–301. [Google Scholar] [CrossRef]
- Wang, K.L.C.; Li, H.; Ecker, J.R. Ethylene biosynthesis and signaling networks. Plant Cell 2002, 14, S1311–S1351. [Google Scholar] [CrossRef]
- Dar, T.A.; Uddin, M.; Khan, M.M.A.; Hakeem, K.R.; Jaleel, H. Jasmonates counter plant stress: A Review. Environ. Exp. Bot. 2015, 115, 49–57. [Google Scholar] [CrossRef]
- Allmann, S.; Halitschke, R.; Schuurink, R.C.; Baldwin, I.T. Oxylipin channelling in Nicotiana attenuata: Lipoxygenase 2 supplies substrates for green leaf volatile production. Plant Cell Environ. 2010, 33, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Halitschke, R.; Baldwin, I.T. Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J. 2003, 36, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Vick, B.A.; Zimmerman, D.C. Pathways of fatty acid hydroperoxide metabolism in Spinach leaf chloroplasts. Plant Physiol. 1987, 85, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Rostás, M.; Ton, J.; Mauch-Mani, B.; Turlings, T.C. Fungal infection reduces herbivore-induced plant volatiles of maize but does not affect naive parasitoids. J. Chem. Ecol. 2006, 32, 1897. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, M.; Wang, Y.; Schuman, M.C.; Weinhold, A.; Schäfer, M.; Baldwin, I.T. Flower-specific jasmonate signaling regulates constitutive floral defenses in wild tobacco. Proc. Natl. Acad. Sci. USA 2017, 114, E7205–E7214. [Google Scholar] [CrossRef] [PubMed] [Green Version]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Q.; Zhou, Y.; Liao, Y.; Zeng, L.; Xu, X.; Jia, Y.; Dong, F.; Li, J.; Tang, J.; Yang, Z. Functional Characterization of An Allene Oxide Synthase Involved in Biosynthesis of Jasmonic Acid and Its Influence on Metabolite Profiles and Ethylene Formation in Tea (Camellia sinensis) Flowers. Int. J. Mol. Sci. 2018, 19, 2440. https://doi.org/10.3390/ijms19082440
Peng Q, Zhou Y, Liao Y, Zeng L, Xu X, Jia Y, Dong F, Li J, Tang J, Yang Z. Functional Characterization of An Allene Oxide Synthase Involved in Biosynthesis of Jasmonic Acid and Its Influence on Metabolite Profiles and Ethylene Formation in Tea (Camellia sinensis) Flowers. International Journal of Molecular Sciences. 2018; 19(8):2440. https://doi.org/10.3390/ijms19082440
Chicago/Turabian StylePeng, Qiyuan, Ying Zhou, Yinyin Liao, Lanting Zeng, Xinlan Xu, Yongxia Jia, Fang Dong, Jianlong Li, Jinchi Tang, and Ziyin Yang. 2018. "Functional Characterization of An Allene Oxide Synthase Involved in Biosynthesis of Jasmonic Acid and Its Influence on Metabolite Profiles and Ethylene Formation in Tea (Camellia sinensis) Flowers" International Journal of Molecular Sciences 19, no. 8: 2440. https://doi.org/10.3390/ijms19082440