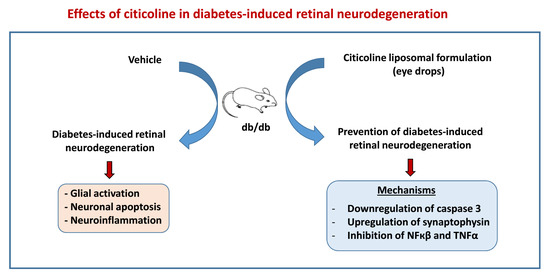
Effects of Liposomal Formulation of Citicoline in Experimental Diabetes-Induced Retinal Neurodegeneration
Abstract
:1. Introduction
2. Results
2.1. Topical Administration of Liposomal Formulation of Citicoline Prevents Retinal Neurodegeneration in Diabetic Mice
2.1.1. Glial Activation
2.1.2. Apoptosis
2.1.3. Electroretinography Abnormalities
2.2. Mechanisms Involved in Neuroprotection
2.2.1. Citicoline Prevents the Downregulation of Synaptophysin Induced by Diabetes
2.2.2. Citicoline Prevents the Upregulation of NF-κB and TNF-α Induced by Diabetes
2.2.3. Citicoline Had No Effect on Extracellular Glutamate Accumulation Induced by Diabetes
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Electroretinogram
4.3. Neurodegeneration Measurements
4.3.1. Measurements of Glial Activation
4.3.2. Apoptosis Assessment
4.4. Methods Used in Order to Explore the Mechanisms of Action
4.4.1. Glutamate Quantification
4.4.2. Assessment of Synaptophysin
4.4.3. Assessment of NF-κB
4.4.4. Proinflammatory Cytokines
4.5. Blood-Retinal Barrier (BRB) Function
4.6. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C.; European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Neurodegeneration is an early event in diabetic retinopathy: Therapeutic implications. Br. J. Ophthalmol. 2012, 96, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C.; European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. [Google Scholar]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Chew, E.; Duh, E.J.; Sobrin, L.; Sun, J.K.; VanderBeek, B.L.; Wykoff, C.C.; Gardner, T.W. Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 412–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simó, R.; Hernández, C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog. Retin. Eye Res. 2015, 48, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L. Citicoline: Update on a promising and widely available agent for neuroprotection and neurorepair. Rev. Neurol. Dis. 2008, 5, 167–177. [Google Scholar] [PubMed]
- Fagone, P.; Jackowski, S. Phosphatidylcholine and the CDP-choline cycle. Biochim. Biophys. Acta 2013, 1831, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Fernández, M.; Rodríguez-Frutos, B.; Fuentes, B.; Vallejo-Cremades, M.T.; Alvarez-Grech, J.; Expósito-Alcaide, M.; Díez-Tejedor, E. CDP-choline treatment induces brain plasticity markers expression in experimental animal stroke. Neurochem. Int. 2012, 60, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sabín, J.; Román, G.C. The role of citicoline in neuroprotection and neurorepair in ischemic stroke. Brain Sci. 2013, 3, 1395–1414. [Google Scholar] [CrossRef] [PubMed]
- Diederich, K.; Frauenknecht, K.; Minnerup, J.; Schneider, B.K.; Schmidt, A.; Altach, E.; Eggert, V.; Sommer, C.J.; Schäbitz, W.R. Citicoline enhances neuroregenerative processes after experimental stroke in rats. Stroke 2012, 43, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Alvarez-Sabín, J.; Castillo, J.; Díez-Tejedor, E.; Ferro, J.; Martínez-Vila, E.; Serena, J.; Segura, T.; Cruz, V.T.; Masjuan, J.; et al. Citicoline in the treatment of acute ischaemic stroke: An international, randomised, multicentre, placebo-controlled study (ICTUS trial). Lancet 2012, 380, 349–357. [Google Scholar] [CrossRef]
- Overgaard, K. The effects of citicoline on acute ischemic stroke: A review. J. Stroke Cerebrovasc. Dis. 2014, 23, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, M.; Yanagi, M. Cytidinediphosphocholine (CDP-choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database Syst. Rev. 2005, 18, CD000269. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, X.A.; Mouzo, R.; Pichel, V.; Pérez, P.; Laredo, M.; Fernández-Novoa, L.; Corzo, L.; Zas, R.; Alcaraz, M.; Secades, J.J.; et al. Double-blind placebo-controlled study with citicoline in APOE genotyped Alzheimer’s disease patients. Effects on cognitive performance, brain bioelectrical activity and cerebral perfusion. Methods Find. Exp. Clin. Pharmacol. 1999, 21, 633–644. [Google Scholar] [PubMed]
- Putignano, S.; Gareri, P.; Castagna, A.; Cerqua, G.; Cervera, P.; Cotroneo, A.M.; Fiorillo, F.; Grella, R.; Lacava, R.; Maddonni, A.; et al. Retrospective and observational study to assess the efficacy of citicoline in elderly patients suffering from stupor related to complex geriatric syndrome. Clin. Interv. Aging 2012, 7, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Cotroneo, A.M.; Castagna, A.; Putignano, S.; Lacava, R.; Fantò, F.; Monteleone, F.; Rocca, F.; Malara, A.; Gareri, P. Effectiveness and safety of citicoline in mild vascular cognitive impairment: The IDEALE study. Clin. Interv. Aging 2013, 8, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Manni, G.; Colacino, G.; Bucci, M.G. Cytidine-5′-diphosphocholine (citicoline) improves retinal and cortical responses in patients with glaucoma. Ophthalmology 1999, 106, 1126–1134. [Google Scholar] [CrossRef]
- Roberti, G.; Tanga, L.; Parisi, V.; Sampalmieri, M.; Centrofanti, M.; Manni, G. A preliminary study of the neuroprotective role of citicoline eye drops in glaucomatous optic neuropathy. Indian J. Ophthalmol. 2014, 62, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Centofanti, M.; Ziccardi, L.; Tanga, L.; Michelessi, M.; Roberti, G.; Manni, G. Treatment with citicoline eye drops enhances retinal function and neural conduction along the visual pathways in open angle glaucoma. Graefe Arch. Clin. Exp. Ophthalmol. 2015, 253, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Roberti, G.; Tanga, L.; Michelessi, M.; Quaranta, L.; Parisi, V.; Manni, G.; Oddone, F. Cytidine 5′-Diphosphocholine (Citicoline) in glaucoma: Rationale of its Use, Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2015, 16, 28401–28417. [Google Scholar] [CrossRef] [PubMed]
- Ottobelli, L.; Manni, G.L.; Centofanti, M.; Iester, M.; Allevena, F.; Rossetti, L. Citicoline oral solution in glaucoma: Is there a role in slowing disease progression? Ophthalmologica 2013, 229, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, S.; Preziosa, C.; Capuano, V.; Spinello, A.; Zucchiatti, I.; Gabellini, D.; Lattanzio, R.; Bandello, F.; Zerbini, G. In vivo evaluation of retinal and choroidal structure in a mouse model of long-lasting diabetes. Effect of topical treatment with citicoline. J. Ocul. Dis. Ther. 2015, 3, 1–8. [Google Scholar]
- Steven, D.W.; Alaghband, P.; Lim, K.S. Preservatives in glaucoma medication. Br. J. Ophthalmol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Grieb, P. Neuroprotective properties of citicoline: Facts, doubts and unresolved issues. CNS Drugs 2014, 28, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, A.; Varano, M.; Gaddini, L.; Mallozzi, C.; Villa, M.; Pricci, F.; Malchiodi-Albedi, F. Neuroprotective effects of citicoline in in vitro models of retinal neurodegeneration. Int. J. Mol. Sci. 2014, 15, 6286–6297. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P. Targeting intraocular neovascularization and edema-one drop at a time. N. Engl. J. Med. 2008, 359, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Leo, L.F.; McGregor, C.; Grivitishvili, A.; Barnstable, C.J.; Tombran-Tink, J. Pigment epithelium-derived factor (PEDF) peptide eye drops reduce inflammation, cell death and vascular leakage in diabetic retinopathy in Ins2(Akita) mice. Mol. Med. 2012, 18, 1387–1401. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; García-Ramírez, M.; Corraliza, L.; Fernández-Carneado, J.; Farrera-Sinfreu, J.; Ponsati, B.; González-Rodríguez, Á.; Martínez-Valverde, Á.; Simó, R. Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes. Diabetes 2013, 62, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Bogdanov, P.; Corraliza, L.; García-Ramírez, M.; Solà-Adell, C.; Arranz, J.A.; Arroba, A.I.; Valverde, A.M.; Simó, R. Topical Administration of GLP-1 Receptor Agonists Prevents Retinal Neurodegeneration in Experimental Diabetes. Diabetes 2016, 65, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Bogdanov, P.; Solà-Adell, C.; Sampedro, J.; Valeri, M.; Genís, X.; Simó-Servat, O.; García-Ramírez, M.; Simó, R. Topical administration of DPP-IV inhibitors prevents retinal neurodegeneration in experimental diabetes. Diabetologia 2017, 60, 2285–2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurihara, T.; Ozawa, Y.; Nagai, N.; Shinoda, K.; Noda, K.; Imamura, Y.; Tsubota, K.; Okano, H.; Oike, Y.; Ishida, S. Angiotensin II type 1 receptor signaling contributes to synaptophysin degradation and neuronal dysfunction in the diabetic retina. Diabetes 2008, 57, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Spiwoks-Becker, I.; Vollrath, L.; Seeliger, M.W.; Jaissle, G.; Eshkind, L.G.; Leube, R.E. Synaptic vesicle alterations in rod photoreceptors of synaptophysin-deficient mice. Neuroscience 2001, 107, 127–142. [Google Scholar] [CrossRef]
- Kwon, S.E.; Chapman, E.R. Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron 2011, 70, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, O.; Hernández-Jiménez, M.; Zarruk, J.G.; Cuartero, M.I.; Ballesteros, I.; Camarero, G.; Moraga, A.; Pradillo, J.M.; Moro, M.A.; Lizasoain, I. Citicoline (CDP-choline) increases Sirtuin1 expression concomitant to neuroprotection in experimental stroke. J. Neurochem. 2013, 126, 819–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Jiménez, M.; Hurtado, O.; Cuartero, M.I.; Ballesteros, I.; Moraga, A.; Pradillo, J.M.; McBurney, M.W.; Lizasoain, I.; Moro, M.A. Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke 2013, 44, 2333–2337. [Google Scholar] [CrossRef] [PubMed]
- Aveleira, C.A.; Lin, C.M.; Abcouwer, S.F.; Ambrósio, A.F.; Antonetti, D.A. TNF-α signals through PKCζ/NF-κB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes 2010, 59, 2872–2882. [Google Scholar] [CrossRef] [PubMed]
- Solà-Adell, C.; Bogdanov, P.; Hernández, C.; Sampedro, J.; Valeri, M.; Garcia-Ramirez, M.; Pasquali, C.; Simó, R. Calcium Dobesilate Prevents Neurodegeneration and Vascular Leakage in Experimental Diabetes. Curr. Eye Res. 2017, 42, 1273–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdanov, P.; Corraliza, L.; Villena, J.A.; Carvalho, A.R.; Garcia-Arumí, J.; Ramos, D.; Ruberte, J.; Simó, R.; Hernández, C. The db/db mouse: A useful model for the study of diabetic retinal neurodegeneration. PLoS ONE 2014, 9, e97302. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Holder, G.E.; Seeliger, M.W.; Yamamoto, S. International Society for Clinical Electrophysiology of Vision (2004 update). Doc. Ophthalmol. 2004, 108, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.J.; Watts, H.R.; Hille, C.J.; Philpott, K.L.; Clark, P.; Gentleman, M.C.S.; Jen, L.S. Glial and endothelial blood-retinal barrier responses to amyloid-beta in the neural retina of the rat. Clin. Ophthalmol. 2008, 2, 801–816. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.B.; Ditewig-Meyers, G.; Graham, K.S.; Scott, R.; Bennett, M.J. Measurement of plasma amino acids by Ultraperformance® Liquid Chromatography. Clin. Chem. Lab. Med. 2011, 49, 1177–1185. [Google Scholar] [CrossRef] [PubMed]








| Target Molecule | Clone | Dilution | Manufacturer |
|---|---|---|---|
| GFAP | Rabbit polyclonal | 1/500 | Abcam (ab7260) |
| Cleaved caspase 3 | Rabbit polyclonal | 1/200 | Abcam (ab3623) |
| Synaptophysin | Rabbit monoclonal | 1/100 | Abcam (ab32127) |
| GLAST | Rabbit polyclonal | 1/100 | Abcam (ab416) |
| IL-1β | Rabbit polyclonal | 1/100 | Abcam (ab9722) |
| TNF-α | Mouse monoclonal | 1/100 | Abcam (ab8348) |
| Serum Albumin | Sheep polyclonal | 1/500 | Abcam (ab8940) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanov, P.; Sampedro, J.; Solà-Adell, C.; Simó-Servat, O.; Russo, C.; Varela-Sende, L.; Simó, R.; Hernández, C. Effects of Liposomal Formulation of Citicoline in Experimental Diabetes-Induced Retinal Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 2458. https://doi.org/10.3390/ijms19082458
Bogdanov P, Sampedro J, Solà-Adell C, Simó-Servat O, Russo C, Varela-Sende L, Simó R, Hernández C. Effects of Liposomal Formulation of Citicoline in Experimental Diabetes-Induced Retinal Neurodegeneration. International Journal of Molecular Sciences. 2018; 19(8):2458. https://doi.org/10.3390/ijms19082458
Chicago/Turabian StyleBogdanov, Patricia, Joel Sampedro, Cristina Solà-Adell, Olga Simó-Servat, Carla Russo, Luisa Varela-Sende, Rafael Simó, and Cristina Hernández. 2018. "Effects of Liposomal Formulation of Citicoline in Experimental Diabetes-Induced Retinal Neurodegeneration" International Journal of Molecular Sciences 19, no. 8: 2458. https://doi.org/10.3390/ijms19082458