Stoichiometric Conditions
Probably the most potential emerging technology, called “close coupled catalysts”, makes use of complex Ce-Zr mixed oxide promoter supported on alumina and of Pd as the active component. Ce-Zr replaces ceria (CeO2) and Pd replaces bimetallic Pt/Rh in traditional TWC formulations. The improved catalytic properties derived from the use of the more refractory Ce-Zr component, which allows mounting of the catalytic converter closer to the exhaust port of the engine, are related to decreasing pollutant concentration during warm-up, i.e. the first minutes of motor operation, where present automobiles produce nearly 50 % of the total emissions. Pd contributes to the task due to its excellent oxidation capability to convert HCs and CO at low temperature (warm-up) and has increased practical application due to the lowering of sulfur levels in current gasolines (with respect to the situation of few years ago).
Ce-Zr component. The structural/morphological properties of the Ce-containing materials are very important to interpret the catalytic/chemical behavior of TWCs and now are well established. Cerium oxides, particularly ceria, are unique as efficient oxygen buffers for damping changes in exhaust gas composition around the oxidant/reductant ratio (stoichiometric point) at which the catalyst works efficiently [
2]. The introduction of other metal atoms in the fluorite-type structure of ceria is usually not complicated; in fact, Zr can be introduced at least up to equimolar proportion [
3], while other cases, like Ca, have a somewhat lower solubility level, close to a 33 at. % [
4].
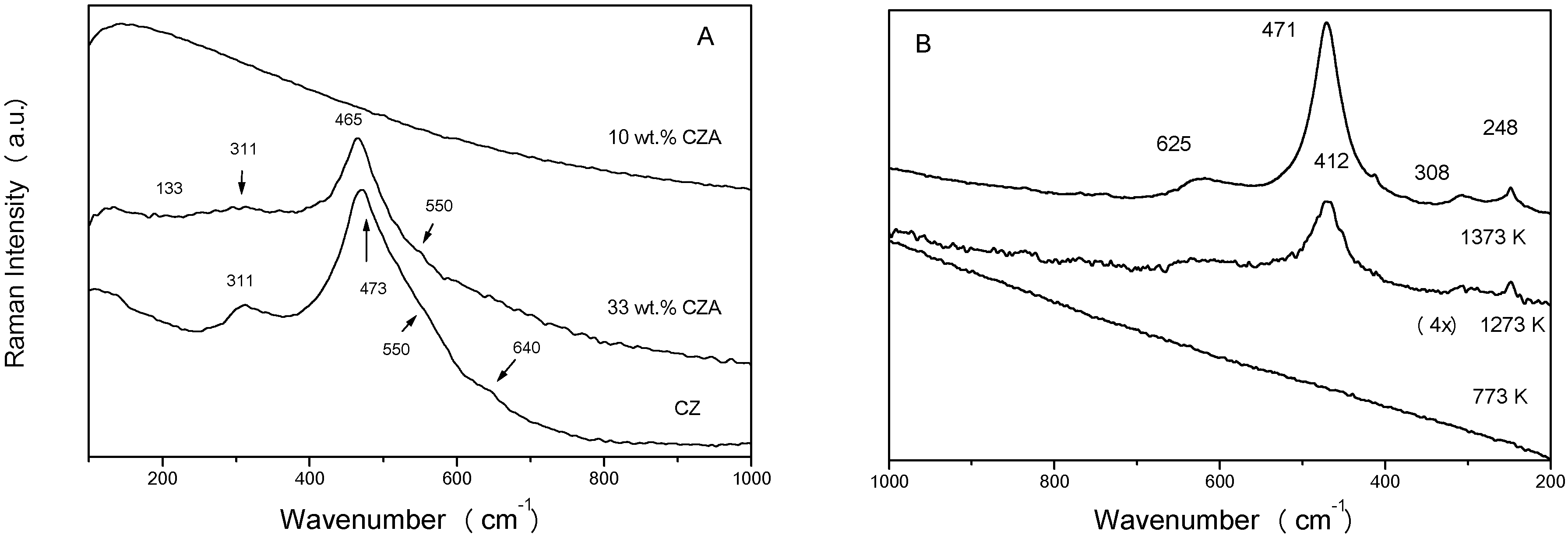
Zr seems however unique in promoting certain properties of ceria, this being partially attributed to the moderate structural modification of the fluorite (Fm3m symmetry group) lattice. Raman spectra (using the 415 nm Ar line as excitation source) indicate the presence of tetragonal phases for bulk nano-sized Ce-Zr mixed oxides (
Fig. 1A). The t´´ phase, found for Ce:Zr atomic ratios close to 1, presents null tetragonal distortion of the unit cell (c/a=1) and differs from fluorite only in a small displacement of the oxygen sub-lattice positions [
3]. Actually, the t´´ phase is a metastable one, whose existence is ascribable to surface energy effects in turn related to the nanosized characteristics of the catalytic materials.
Fig. 1.
Raman Spectra of CZA systems: A) with different CZ loading; B) 10 wt.% CZ on alumina aged at different temperatures.
Fig. 1.
Raman Spectra of CZA systems: A) with different CZ loading; B) 10 wt.% CZ on alumina aged at different temperatures.
A more complicated situation occurs when the Ce-Zr promoter is supported on alumina. Alumina is introduced to confer high surface area as well as thermal and mechanical resistance to the TWC system. The preparation of this complex support requires special methods. Complex CZA supports with the Ce-Zr component having a tetragonal t´´ symmetry (identified by its Raman features,
Fig. 1A) can be obtained by using especialized preparation methods, like those based on inverse microemulsions [
5]. In such cases, the ability to keep a high dispersion of the promoter on the carrier has particular importance if the catalytic converter is exposed to high temperature. Close coupled converters can reach up to 1373 K. X-ray diffraction (XRD –data not shown) gives evidence that CZA supports maintain structural integrity up to 1273 K but above this cut-off temperature, disproportionation in two phases (Ce and Zr-rich, respectively) can occur. At the moment, no detailed explanation has been put forward to interpret such result although it is clear that control of the surface Ce:Zr atomic ratio (in turn commanded by the Ce-Zr/Al
2O
3 interaction) is very important to minimize phase segregation [
6]. In any case, after a 1373 K aging treatment, the dominant Ce-containing phase still has tetragonal structure (
Fig. 1B), likely conferring adequate oxygen handling properties to the material.
The segregation phenomenon detected above 1273 K has several consequences as illustrated nicely by transmission electron microscopy (TEM). At the top of
Fig. 2, that displays an aggregate of the CZA material (aged at 1373 K), the absence of Ce-Zr induces the presence of low surface α-Al
2O
3, a single crystallite of which is detected by electron diffraction (ED –inset a in
Fig. 2). At the bottom, the presence of the promoter (evidenced by EDS and the observation of a large Ce-Zr crystallite -ED at the right; inset b in
Fig. 2) assists in the stabilization of the high surface θ-Al
2O
3 phase (ED at the left; inset c in
Fig. 2). In addition to maintaining the carrier surface area, which reduces losses of the promoter and active metal by covering of such components with the carrier, Ce-Zr itself suffers less deactivation (when compared with ceria) by sintering as a function of aging temperature. In
table 1, a comparison is made between CZA and standard CA (ceria/alumina) supports, both prepared by microemulsion. Average particle size indicates the higher stabilization of the mixed oxide promoter up to 1273 K, the situation being less clear above this temperature [
6].
Table 1.
Average particle size (measured by TEM, in nm) of the promoter component in CZA and CA specimens after aging treatments under air.
Table 1.
Average particle size (measured by TEM, in nm) of the promoter component in CZA and CA specimens after aging treatments under air.
| Aging Treatment | CZA | CA |
|---|
| 773 K | 2-3 | 4 |
| 1273 K | 10 | 20 |
| 1373 K | 26,18a | 20 |
As mentioned, a surface property (the surface Ce:Zr atomic ratio) of the promoter particles has an influence on the structural behavior upon aging. Moreover, the catalytic properties are directly connected with surface properties. The adsorption of methanol followed by Infrared of the C-O stretching region identifies the presence of both Zr (band at 1160-1155 cm
-1) and Ce (1105-1090 cm
-1) at the surface of the Ce-Zr component, providing evidence of the true mixed oxide nature at the surface and showing a progressive decrease in Zr content with the decrease of the (Ce,Zr) loading (
Fig. 3) [
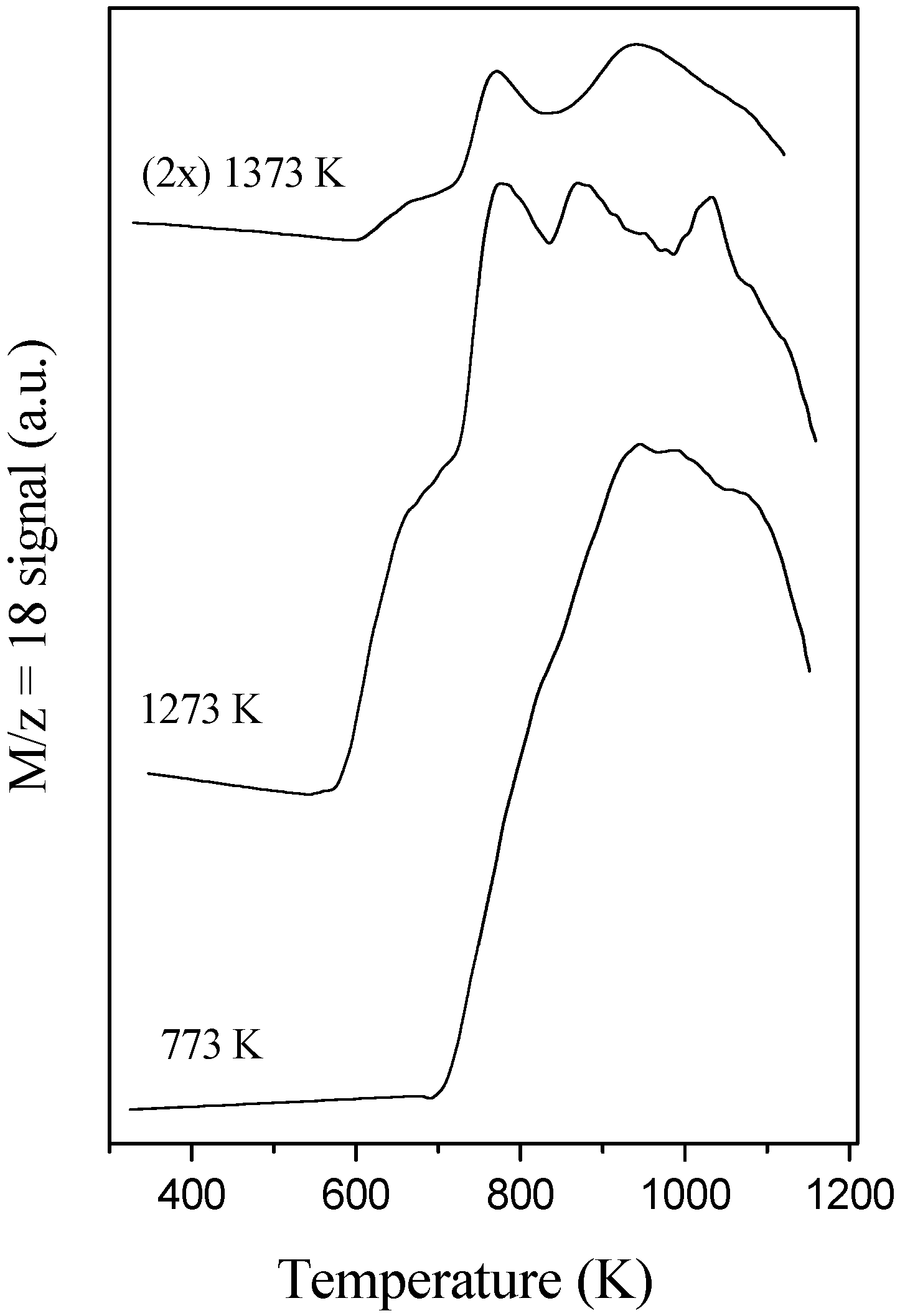
7]. The oxygen storage properties of the support are however a function of both surface and bulk properties; analysis of temperature-programmed reduction (TPR) profiles (
Fig. 4) confirms this, showing a continuous reduction process of the calcined materials. On the other hand, severe aging of these supports (above 1273 K) decrease the overall capacity of labile oxygen although additional low temperature (650 K) reduction peaks appear. This latter feature, which denotes more facile mobility of oxygen on large (> 10nm) Ce-Zr particles, can be associated with the excellent buffer properties presented by CZA samples under working conditions [
6].
Fig. 2.
Bright-field TEM image of a CZA sample calcined at 1100 K. Insets display local expanded views and their corresponding ED patterns: a) α-Al2O3 (42 zone axis), b) Ce0.67Zr0.33O2 ( zone axis) and c) θ-Al2O3.
Fig. 2.
Bright-field TEM image of a CZA sample calcined at 1100 K. Insets display local expanded views and their corresponding ED patterns: a) α-Al2O3 (42 zone axis), b) Ce0.67Zr0.33O2 ( zone axis) and c) θ-Al2O3.
Fig. 3.
FTIR spectra after methanol adsorption on samples outgassed at 773 K, followed by outgassing at 373 K. CZA samples have the alumina contribution subtrated.
Fig. 3.
FTIR spectra after methanol adsorption on samples outgassed at 773 K, followed by outgassing at 373 K. CZA samples have the alumina contribution subtrated.
Fig. 4.
TPR-MS traces of H2O (M/z 18) of a 10 wt. % CZA support aged at different temperatures.
Fig. 4.
TPR-MS traces of H2O (M/z 18) of a 10 wt. % CZA support aged at different temperatures.
Active metal component. Although some recent TWC formulations use Pd as the active component, the significant recent reduction in S-level in gasoline has allowed attention to be directed to base metals such as Cu [
8], which may be as active as noble metals while using gasoline with S-content below 10 ppm (a level which may be in use the next decade in USA).
In the case of current Pd-based TWC systems, studies show that the active sites are mainly located at the metal-promotor interface [
9,
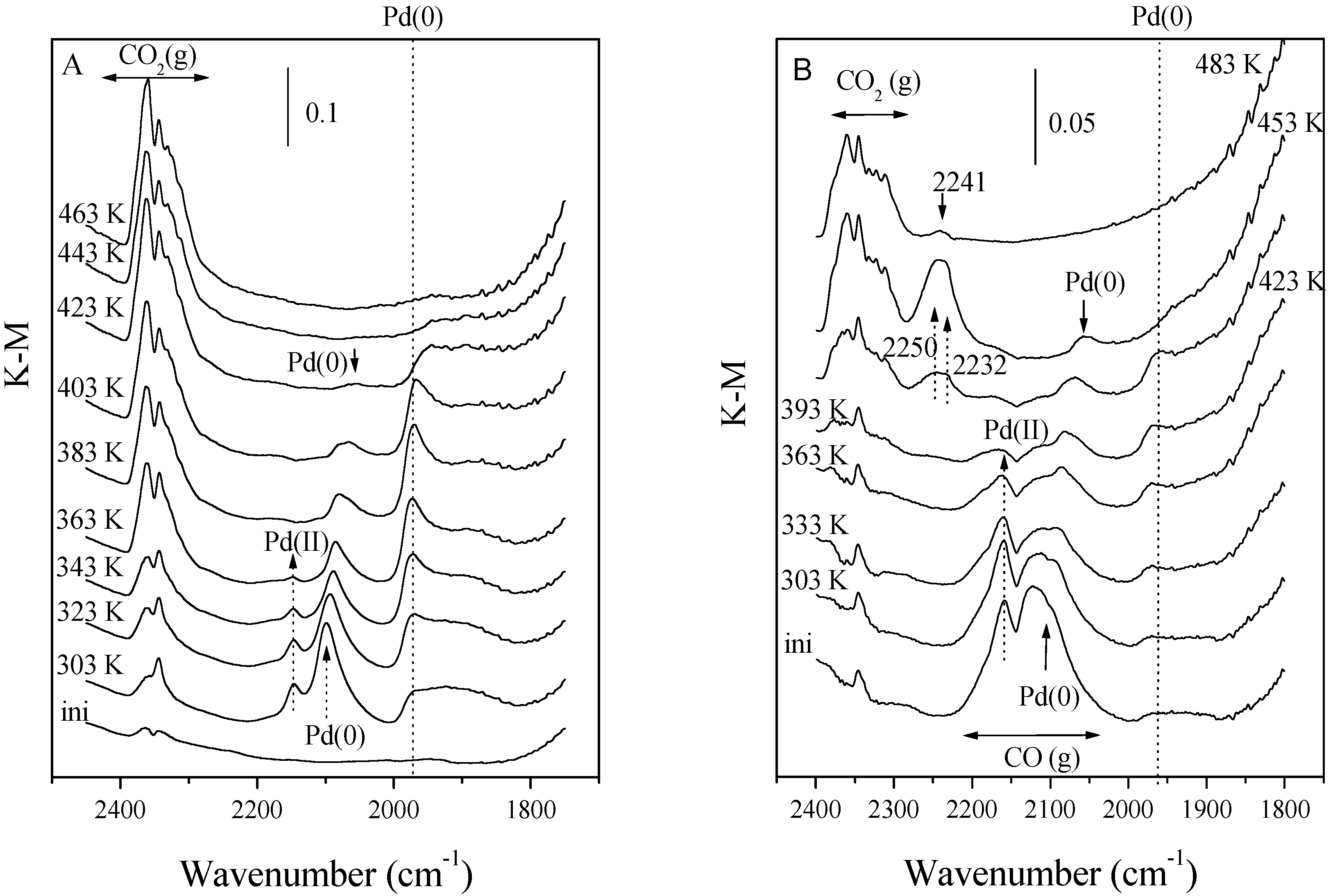
10]. For CO oxidation, the most efficient centers are proposed to be located at the interface with 3D-like (tridimensional) Ce-Zr particles and require the presence of metallic Pd (as shown by IR data presented in
Fig. 5A) to activate the CO molecule [
9]. For NO reduction, the efficient centers are located at the interface with 2D Ce-Zr particles (i.e. promotor particles structurally and/or electronically affected by the carrier) and here the metal surface is (partially) passivated by the presence of N/O species originating from NO dissociation (
Fig. 5B) [
10]. The difference between both types of interface sites is mainly related to the ease of the reduction (vacancy creation) process of the Ce-Zr promoter and its influence on the metal redox state, which needs to be shifted to a more reduced level in the case of NO reduction centers [
9,
10].
Fig. 5.
DRITFS spectra of a PdZCA specimen during reaction: A) CO+O2, and B) CO+NO+O2, at increasing temperatures.
Fig. 5.
DRITFS spectra of a PdZCA specimen during reaction: A) CO+O2, and B) CO+NO+O2, at increasing temperatures.
The presence of HCs in the exhaust gas mixture decreases the structural sensitivity of the CO oxidation and NO reduction reactions; no significant optimization is observed on the light-off curves of both elimination reactions as a function of the metal-promotor interface properties. This implies, for example, that aging treatments do not have a strong influence on catalyst performance as long as the Ce-Zr promoter maintains structural/phase integrity. At least at moderate spatial velocities, this insensitivity is attributed to the low structural sensitivity of the desorption process of HC fragments from the interface, which acts as a self-poison for the CO/NO elimination reactions [
11]. This problem may be resolved by the use of multilayer formulations, using a first (higher loaded) Pd/Al
2O
3 layer which partially handles NOx removal, the most demanding reaction in the case of Pd-based systems, and a second layer of PdCZA which eliminates the remaining pollutants. An alternative route is the retention of HCs at low temperature (warm-up) using porous trapping materials, in order to allow PdZCA to reach its optimum CO/NO
x elimination potential [
12].
Another approach used to improve Pd activity, particularly in the NOx elimination, involves introduction of a second metal (preferably cheap and widely available) that can modulate the properties of Pd by alloying. Among several options, we have investigated the use of Cu as previous theoretical analysis provide evidence of electronic modification of Pd centers, which become negatively charged (with respect to the bare metal) inducing a strong enhancement on N-O bond activation and dissociation [
13]. However, as shown in
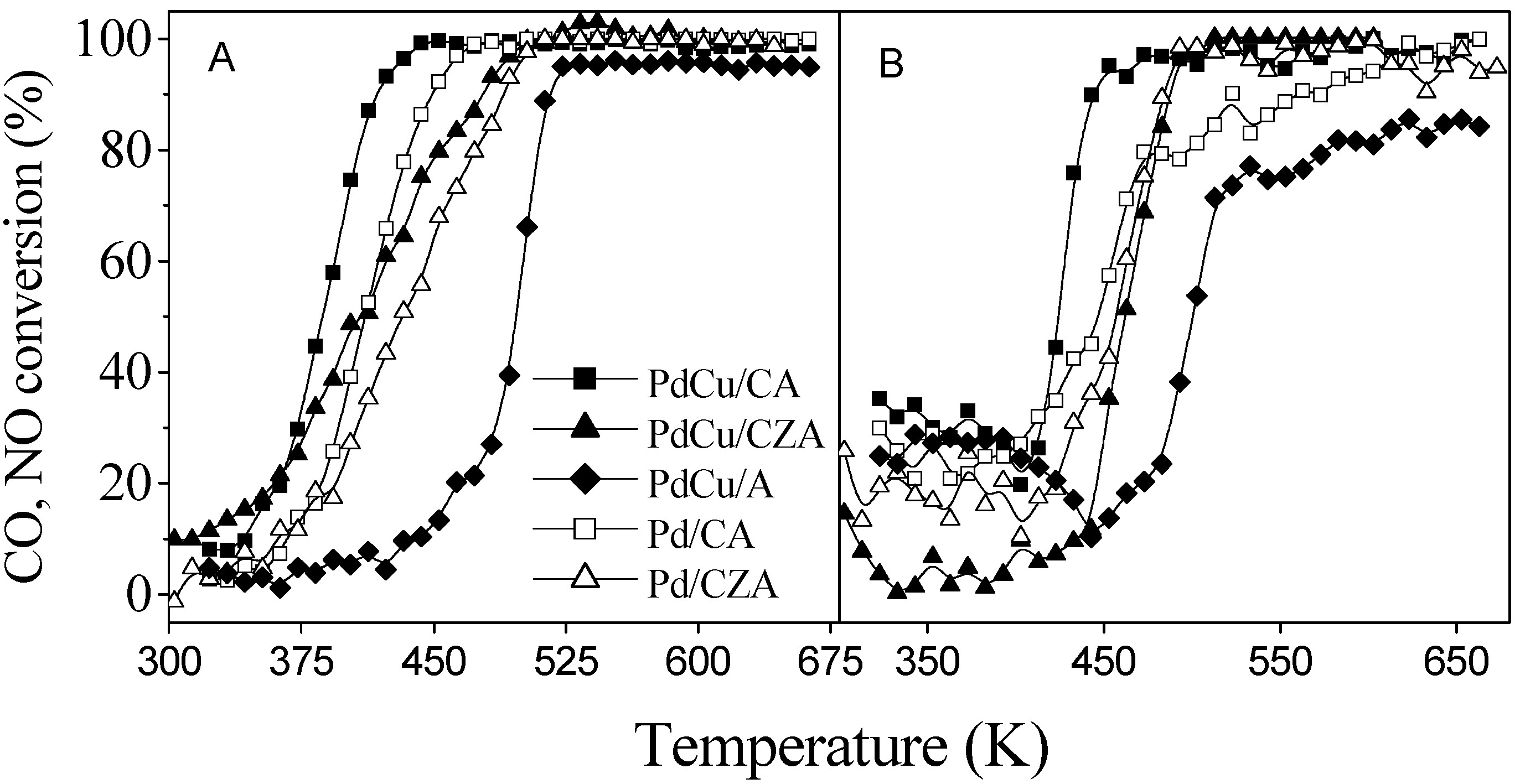
Fig. 6, while the presence of Cu was indeed able to promote CO oxidation and NO reduction on classical CA-based catalysts, in the case of CZA-based systems this beneficial effect is absent [
14]. A Pd K-edge XANES study of the ZCA-based specimen using statistical tools (which allows information to be obtained concerning the nature and concentration of Pd-containing phases) allows an interpretation of such phenomenology: although the onset of Pd-Cu alloy occurs at the beginning of the light-off curve in parallel with metallic Pd, it is disrupted at higher temperature, yielding metallic Pd, which is the only noble metal containing phase at temperatures corresponding with those where complete conversion of pollutants takes place. As Pd-Cu alloys are thermodynamically stable with respect to the parent metals, interaction of Cu with the Ce-Zr promoter (but not with ceria alone), probably resulting in formation of a ternary phase, is likely the cause of this behavior [
15].
Fig. 6.
A) CO and B) NO elimination profiles of Pd and Pd-Cu ZCA and CA supported systems.
Fig. 6.
A) CO and B) NO elimination profiles of Pd and Pd-Cu ZCA and CA supported systems.
Lean Conditions
The possibility of having efficient lean condition catalytic converters was first considered following studies of the Cu-ZSM-5 system. Later analyses of other Cu based systems showed that those supported on alumina present similar activity (if adequately prepared) and eliminated problems associated with structural collapse owing to the presence of water in the gas exhaust stream [
16]. It can be noted, nevertheless, that Cu systems, as well as some other metals as In, Ga or Fe, have their activities somewhat lowered by the presence of water.
The use of alumina as support for this process is now widespread. In fact, it seems to act as an active component, catalyzing some of the reaction mechanism steps. Lean condition catalysts can be classified in two families; those containing noble metals as the active component and those with base metals. Noble metals, typically Pt, have limited possibilities of wide-scale application due to the high N
2O yield in NOx removal reactions and to a narrow operation temperature window [
17]. Base metal catalysts do not present these particular problems but show activity only above 623 K, limiting their potential. In order to overcome the mentioned limitation, intensive work has been performed over the last few years in order to understand the chemical basis of the behavior exhibited by these systems.
Of the systems which have captured the attention of researchers, three are the base metals, Ag, Co and Sn. In terms of the behavior of the active phase, these catalysts present many similarities. In the case of Ag and Co, the most active catalysts contain dispersed phases such as small oxide-like aggregates, which are stable under reaction conditions. This requirement of highly dispersed phases is based on the knowledge that base metal particles in weak interaction with the support tend to form large metallic-like particles by interaction with the HCs during reaction, leading to combustion of the HC fragments with oxygen in preference to the desired reaction with N-containing species. For Sn, the situation is not so clear but the presence of amorphous phases, which escape detection by diffraction techniques, concurs in the same direction [
18].
As already noted, the main drawback of these catalysts for operation under real conditions is related to poor performance at temperatures below 623 K. Mechanistic studies have been performed in order to clarify the situation. For Ag [
19] and Co [
20] it appears clear that NO activation to nitrate species takes place on the alumina surface (via NO
2 as an intermediate produced by metal activation/gas-phase reaction between NO and O
2 and) but does not significantly affect the reaction rate. However, the activation of HCs into oxygenated intermediates is important as these present higher selective reduction activity. As shown in
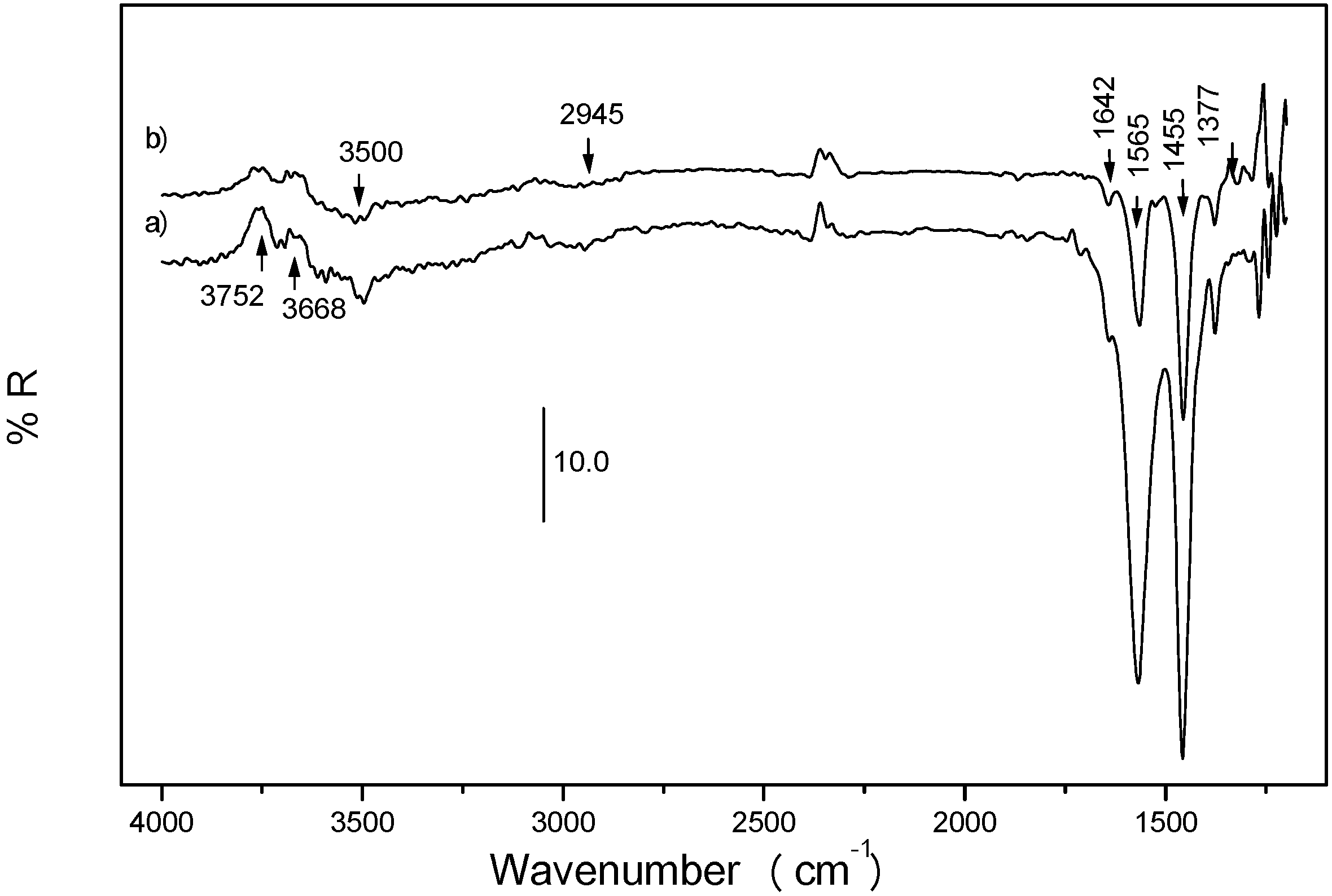
Fig. 7, the treatment of a silver catalyst with NO, previously contacted with HC+O
2, display the presence of infrared bands ascribed to carboxylate groups (1565, 1455 cm
-1) and bands at 1642 and 1377 cm
-1 which, additional experiments confirm, are indicative of the presence of surface oxygenate intermediates with C=C bonds [
19]. The base metal used appears to play an important role in generating the adequate oxygenate(s) and in maximizing its (their) surface concentration, both factors being crucial in determining the observed reaction rate [
21].
Fig. 7.
In-situ DRIFTS difference spectra (i.e. the initial spectrum in O2 subtracted) of 1.5 wt % Ag/Al2O3 systems recorded at 723 K in a) 0.16 % C3H6+ 5 % O2, b) subsequent incorporation of 0.16 % NO to the reactant flow.
Fig. 7.
In-situ DRIFTS difference spectra (i.e. the initial spectrum in O2 subtracted) of 1.5 wt % Ag/Al2O3 systems recorded at 723 K in a) 0.16 % C3H6+ 5 % O2, b) subsequent incorporation of 0.16 % NO to the reactant flow.
The results outlined above strongly suggest that the use of a pre-catalyst for HC activation may then help in enhancing catalytic activity and could enhance the window of operation of base metals to allow operation at lower temperatures. Nevertheless, this may not be enough and multimetallic systems, which contain noble metals in a separate layer (i.e. avoiding contact with the base metal), may need to be included in industrial formulations in order to provide adequate catalytic activity already at 473 K and reach relatively wide temperature windows.
Oscillating Lean-Stoichiometric Conditions
Lean-burn operation reduces fuel consumption and thus reduces the CO
2, CO and HC emitted during driving. As it appears that there are problems in obtaining adequate catalytic system for lean conditions operation, the use of engine management systems, which allow alternate operation under lean and stoichiometric (or rich) conditions, is seen as an alternative to go some way to achieving the previously mentioned objectives. In this case, nitrogen oxides constitute the main pollutant group and TWC-derived PtBa systems have been proposed to provide the so-called NO
x storage-reduction systems. The elimination of NO
x is then reached in two steps; under lean conditions these species are stored on the surface of the alumina and the baria component. The latter changes its chemical state depending on the gaseous atmosphere present, and is introduced in the formulation to capture NO
x forming Ba nitrate. During a second step, under stoichiometric conditions, HCs/CO/H
2 reactants are used to reduce the stored NO
x species to N
2[
12].
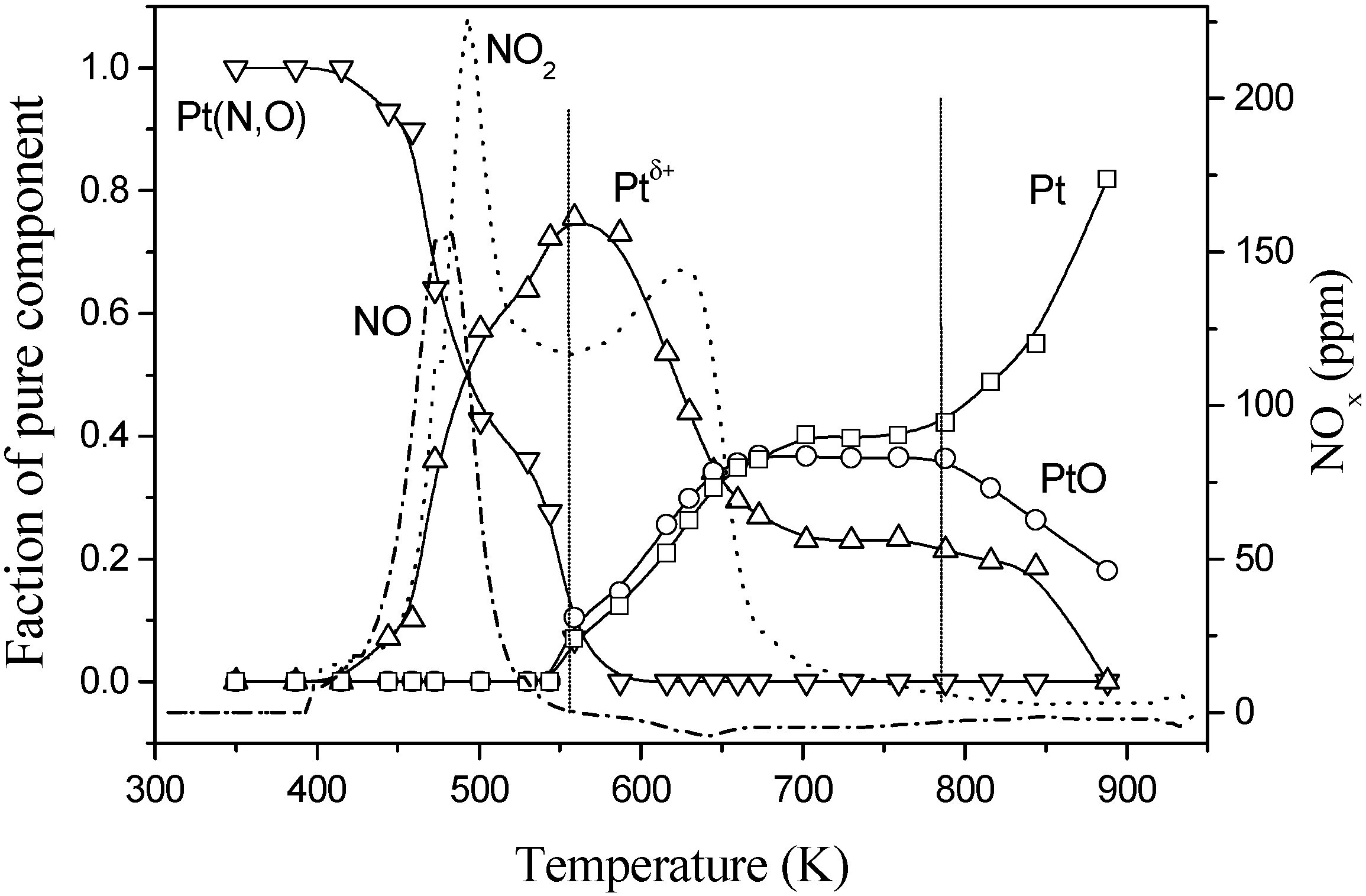
Fig. 8.
Pt concentration and NOx desorption profiles of a NO+O2 pretreated Pt/BaO/Al2O3 catalyst during a temperature ramp under a stoichiometric C3H6+O2 stream.
Fig. 8.
Pt concentration and NOx desorption profiles of a NO+O2 pretreated Pt/BaO/Al2O3 catalyst during a temperature ramp under a stoichiometric C3H6+O2 stream.
The storage of NO
x is a rather complex phenomenon where competition between the formation of barium carbonate (produced from CO
2) and nitrate, which the latter is favored only above 673 K, is observed. The chemical/physical properties of the Ba component deposited on the alumina are critical; a combined XANES/XRD study illustrated that only a few near-surface layers are involved in the formation of an amorphous barium nitrate phase, suggesting that the dispersion of the Ba component plays an important role in optimizing storage properties [
22,
23]. Nevertheless, the most complicated aspect in the elimination of NO
x is related to its reduction step. Although the active metal contribution is indispensable in obtaining the reduced products, it is not clear how the different components of the system interacts during reduction. In
Fig. 8, analysis of the Pt state (concentration profiles extracted from a XANES analysis of Pt L
III-edge) as well as the NO
x desorption products during a ramp under a stoichiometric HC+O
2 mixture for a catalyst pretreated with NO
2+O
2 at 673 K is presented. It is clear that NO/NO
2 evolution is observed from the catalyst at low temperature where only oxidized states of Pt (corresponding to a Pt phase which contains N and O as ligands and a partially reduced Pt compound) are present. It appears that absence of Pt(0) will probably limit efficient operation until 550 K in the case of Pt-alone systems. Above such a cut-off temperature, the presence of this metallic-like Pt phase allows the formation of N
2 although the evolution of other oxidized N-species is also detected [
24]. In any case, the system behavior in the two steps, storage and reduction, is further complicated when sulfur (present in gasoline) is taken into account, as barium sulfate formation is favored with respect to other products and this compound is difficult to eliminate during the reduction step, requiring extended operation of the engine under stoichiometric or rich conditions and elimination of sulfur as H
2S.
Until recently, the most effective catalyst required the addition of Rh to the formulation, inducing several beneficial effects but incrementing significantly the price of the catalyst. Firstly, Rh helps to reduce sulfur sensitivity by producing a more effective regeneration of the Ba component during the stoichiometric part of the cycle. The second beneficial effect is related to an increment in the yield to N2 on the reduction of NOx stored species, a well known fact from experience with TWC. In the future, it is expected that chemical modification of the Ba component by introducing other co-cations can help in improving the performance of these catalytic systems, allowing the removal of Rh from the formulation.