1. Introduction
Well-known in jewelry as sapphire, α-Al
2O
3 (
corundum) nowadays is one of the most widespread ceramic materials [
1]. It is used in particular, as an advanced substrate for ultrathin metal film deposition [
2]. Alumina-supported transition metal catalysts are of continually growing interest due to their high efficiency in several technologically important reactions including oxidation of CO and hydrogenolysis [
3]. In addition to the widespread use of γ
-Al
2O
3 as a substrate both stoichiometric and reduced α
-Al
2O
3 are also used for this purpose [
4]. Moreover, metallic species supported on a corundum substrate, in particular Pd/α
-Al
2O
3 [
5], are used quite effectively as model catalysts.
It is generally believed that the Al-terminated surface is energetically favored over the O-terminated one, since the former is stoichiometric and closer to the bulk structure [
6]. Nevertheless, the O-terminated corundum surface has been observed experimentally as well [
7]. Theoretical studies [
8,
9] indicate that even under conditions of high oxygen gas partial pressure the Al-terminated corundum surface is stable (contrary to conclusions of the review paper [
10]), while even a small hydrogen concentration on the α-Al
2O
3(0001) substrate stabilizes considerably the alternative O-terminated surface. The lower stability of the O-terminated substrate as compared to the Al-terminated one, as well as the presence of O-termination on the defective α-Al
2O
3 (0001) surface (containing either steps or flat areas in the vicinity of vacancies in the outermost layer of the Al
3+ sublattice [
11]) could be responsible for some conflicting results [
9,
10]. The presence of hydrogen on this substrate and its further hydroxylation could also help to explain the discrepancies between some theoretical estimates [
12,
13,
14,
15] and experimental measurements [
8,
9,
10] concerning the relaxation of the surface and subsurface crystalline layers of pure corundum.
In our previous investigation of Ag adsorption sites on α-Al
2O
3(0001) [
16], using the periodic Hartree-Fock method with
a posteriori electron correlation corrections (hereafter
HF-CC method), we found
two favorable adsorption sites for 1/3 ML silver coverage of the O-terminated α-Al
2O
3(0001) substrate (one Ag atom per three O
2− outermost ions, as shown in
Fig. 1 and
Fig. 2a) optimized earlier by Puchin
et al. [
13]. These are:
- (i)
Over large equilateral oxygen triangles, where Ag atoms replace surface Al
3+ ions on an Al-terminated surface; hereafter we call these sites
E2 (closest positions to equilateral surface oxygen triangles shown in
Fig. 2a) in contrast to more remote sites
E1 (in
Fig. 2b),
- (ii)
Over non-equilateral surface oxygen triangles, which lie atop the oxygen ions of the next subsurface oxygen layer; we call these sites H (hollow), because there are no Al3+ ions below these triangles.
Adhesion energies per Ag atom for both sites on the O-terminated surface were estimated to be about 3 eV [
16], so that such adhesion cannot be considered as physisorption, the more so because the adsorbed silver atoms become ionized into Ag
+ ions. Other possible adsorption sites, including Ag positions on top of the outermost O
2− ions, were found to be energetically less favorable.
Verdozzi
et al. [
17] have modeled Ag and Pt adhesion to an Al-terminated corundum slab which included 18 oxygen (0001) planes, using Density Functional Theory (DFT) calculations with plane-wave pseudopotentials. Their general conclusion was that the interaction between silver atoms and substrate in the Ag/corundum interface is physisorption, although well-separated metal atoms (1/3 ML) may be more strongly bound due to the effect of the surface Madelung potential. However, they neglected metal adhesion on an O-terminated surface. Similar DFT plane-wave studies were performed for Nb adhesion [
18] as well as for Al and Cu adhesion [
19] on both the Al- and O-terminated surfaces of a corundum (0001) substrate. It was found that niobium bonding on the O-terminated surface (13 eV per Nb atom) is much stronger than that on the Al-terminated surface (3.4 eV) [
18]. Experimental observations of very strong niobium-corundum adhesion have also been associated with O-termination [
20]. At the same time, unlike noble silver, Nb is a highly reactive metal, so that the outer aluminium ions of the Al-terminated α-Al
2O
3(0001) surface can be substituted by niobium atoms after their adsorption on the corundum substrate. To clarify the nature of noble metal adhesion on corundum (0001) surface, we present in this paper a quantitative analysis of their interfacial bonding.
2. Method and model
As mentioned previously, we used in these calculations the HF-CC method with Gaussian-type basis sets (BSs) as implemented in the computer code CRYSTAL98 [
21]. The Perdew-Wang functional (PWGGA [
22]) was mainly used in a posteriori electron correlation corrections. To perform better CRYSTAL simulations on corundum, Catti et al. [
23] modified the all-electron basis sets used in previous α-Al
2O
3 studies [
12,
13] by introducing a 3d-polarization function in addition to sp-polarization functions and by re-optimizing core and valence shells. Thus, for aluminium and oxygen ions, we use 8(s)-511(sp)-1(d) and 8(s)-411(sp)-1(d) BSs, respectively [
23]. For silver atoms, the same Ag BS was used as earlier [
16], employing the small-core Hay-Wadt pseudopotential for the atomic core [
24] and 311(sp)-31(d) Gaussian-type functions for both valence and virtual shells. The energies quoted in this paper include the BS superposition errors (BSSE) evaluated according to the standard procedure implemented in CRYSTAL98 [
21].
Figure 1.
Top view of the oxygen plane of the corundum (0001) substrate, as optimized in [
13], and two neighboring aluminium planes positioned above and below the oxygen plane (green circles with different internal patterns). In these planes, Al
3+ ions form a hexagonal network with a rhombic unit cell, whereas O
2− ions form a more complicated regular structure containing equilateral triangles of three different sizes. Each may be distinguished by the shade of red color used, the larger the triangle the lighter the shading used. Intermediate versatile triangles are shown in white.
Figure 1.
Top view of the oxygen plane of the corundum (0001) substrate, as optimized in [
13], and two neighboring aluminium planes positioned above and below the oxygen plane (green circles with different internal patterns). In these planes, Al
3+ ions form a hexagonal network with a rhombic unit cell, whereas O
2− ions form a more complicated regular structure containing equilateral triangles of three different sizes. Each may be distinguished by the shade of red color used, the larger the triangle the lighter the shading used. Intermediate versatile triangles are shown in white.
For the simulation of silver adhesion on the corundum substrate, we have used a
slab model, periodic in two dimensions, and of finite thickness in the direction perpendicular to the (0001) plane [
12]. The α-Al
2O
3(0001) substrate belongs to the hexagonal plane group
P321, the optimized length of the side
a in its surface unit cell being 4.76 Å (
Fig. 1). Both a 7-layer slab with outermost oxygen ions (
Fig. 2a) and a 9-layer slab terminated by aluminium ions (
Fig. 2b) have been modeled, using primitive unit cells that contained 13 and 15 atoms, respectively. The
C3v rotation axes normal to the surface contain Al3+ ions and form a regular network normal to a (111) plane of the face-centered cubic (
fcc) structure, this plane being parallel to the (0001) plane. The distance
AA/=
between adjacent Al
3+ axes is 2.75 Å; the two rhombic surface unit cells formed by those axes are shown in
Fig. 1. All the O
2− ions in the bulk corundum are equivalent; its slab structure optimized by Puchin
et al. [
13] forms a periodic network of
equilateral triangles with
b1,
b2 and
b3 sides (2.64 Å, 2.74 Å and 2.87 Å, respectively), as well as
versatile triangles positioned between them (
Fig. 1). The oxygen-containing corundum (0001) planes are equivalent, with an interplanar distance
c ≈ 2.16 Å (
Fig. 2). Each oxygen plane can be transformed into a neighboring one by a synchronous combination of a 60
o rotation around the corresponding
C3v axes and a translation by
AA/ (
Fig. 1), so that the two rhombic surface unit cells (
Fig. 1) coincide. Each oxygen plane is associated with two adjacent, less-densely packed aluminium planes, so that each Al
3+ ion is positioned either above the center of the largest equilateral oxygen triangle or below the middle triangle (at a distance
d of 0.84 Å in bulk corundum).
Figure 2.
Side views of α-Al2O3(0001) slabs where silver atoms at 1/3 ML coverage are distributed regularly on the aluminium C3v axes above and below O-terminated (a) and Al-terminated (b) models, respectively. The interlayer structure of the interface is defined by c, d and zAg parameters. The most probable positions for Ag adsorption have been found to be E1 for (b), E2 for (a), and H.
Figure 2.
Side views of α-Al2O3(0001) slabs where silver atoms at 1/3 ML coverage are distributed regularly on the aluminium C3v axes above and below O-terminated (a) and Al-terminated (b) models, respectively. The interlayer structure of the interface is defined by c, d and zAg parameters. The most probable positions for Ag adsorption have been found to be E1 for (b), E2 for (a), and H.
In this study, we have varied the interfacial distance
zAg for both terminations (
Table 1) and the Al−O plane distance
d for the Al-terminated corundum substrate (its optimized value is analyzed in subsection
3A). To reduce computational effort we exploited the system’s symmetry by applying a two-side adhesion model, with spatially equivalent silver layers on
both surfaces of the corundum slab. For both Al- and O-termination we have considered two different Ag coverages: 1/3 ML (
Fig. 3a) and 1 ML (
Fig. 3b). For a 1/3 Ag ML coverage of the Al-terminated slab, two different adsorption patterns have been modeled: silver atoms over the smallest equilateral oxygen triangles, site
E1 (
Fig. 2b), or Ag over versatile O triangles,
i.e. above 1/3 of the
H sites (
Fig. 3a). For the 1/3 Ag ML coverage of the O-terminated slab, two patterns have been also used: silver atoms above the largest equilateral oxygen triangles, site
E2 (
Fig. 2a), or Ag above 1/3 of the same
H sites. In both cases, silver atoms are distributed regularly on the substrate forming a periodic
superstructure. For 1 Ag ML coverage, two different adsorption patterns have been also considered for both terminations: either above all equilateral triangles or above all the
H sites (
Fig. 3b). Space and symmetry compatibility between the Ag(111) and α-Al
2O
3(0001) planes mentioned above allows us to consider relatively simple models of the Ag/corundum interface without the inclusion of misfit dislocations.
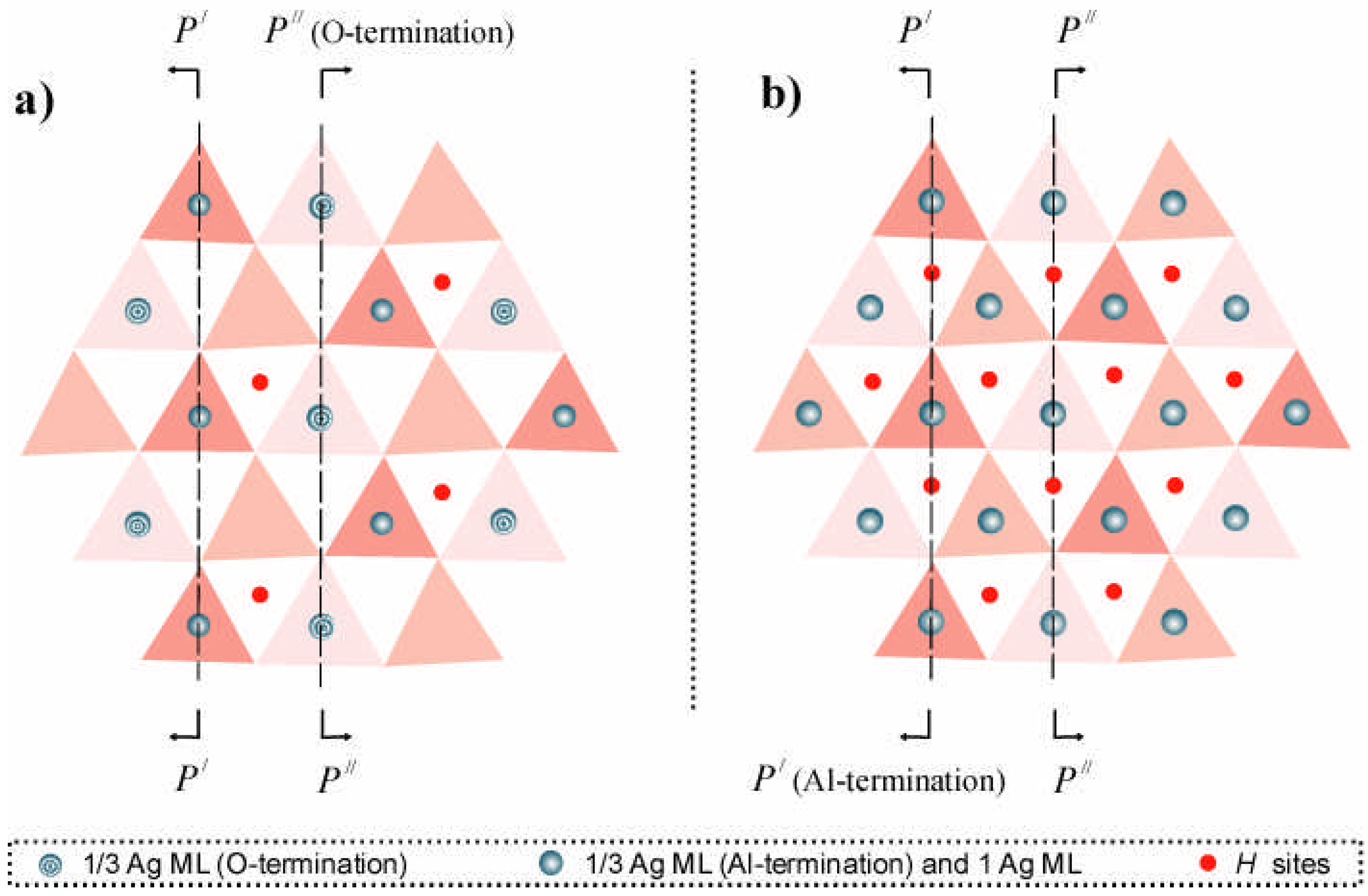
Figure 3.
Top views of Ag atom distributions over Al- and O-terminated corundum (0001) surfaces for the two different coverages: 1/3 ML of the Ag(111) plane (a) and 1 Ag ML (b). The two different silver atom adsorption positions are above equilateral and versatile triangles forming the outermost O plane. Circles with two different internal patterns (a) show the two kinds of Ag atom distributions over equilateral triangles on Al- and O-terminated substrates, whereas positions over versatile triangles are shown by red dots in both (a) and (b). Planes
P′− P′ and
P′′− P′′ are used in
Fig. 4 and
Fig. 5, respectively, to show the electron charge density distributions in cross sections perpendicular to the (0001) surface.
Figure 3.
Top views of Ag atom distributions over Al- and O-terminated corundum (0001) surfaces for the two different coverages: 1/3 ML of the Ag(111) plane (a) and 1 Ag ML (b). The two different silver atom adsorption positions are above equilateral and versatile triangles forming the outermost O plane. Circles with two different internal patterns (a) show the two kinds of Ag atom distributions over equilateral triangles on Al- and O-terminated substrates, whereas positions over versatile triangles are shown by red dots in both (a) and (b). Planes
P′− P′ and
P′′− P′′ are used in
Fig. 4 and
Fig. 5, respectively, to show the electron charge density distributions in cross sections perpendicular to the (0001) surface.
The binding (adhesion) energy
per silver atom at the equilibrium distance,
Ebind (
Table 1), is estimated as a straightforward energy difference:
where
is the total energy for the interface at the equilibrium Ag-corundum slab distance;
E Ag slab and
E corundum slab are the total energies for the corresponding isolated subsystems with the
same geometry as that of the optimized interface system;
k = 2 for the model with two-side adhesion. The change of the silver Mulliken charge,
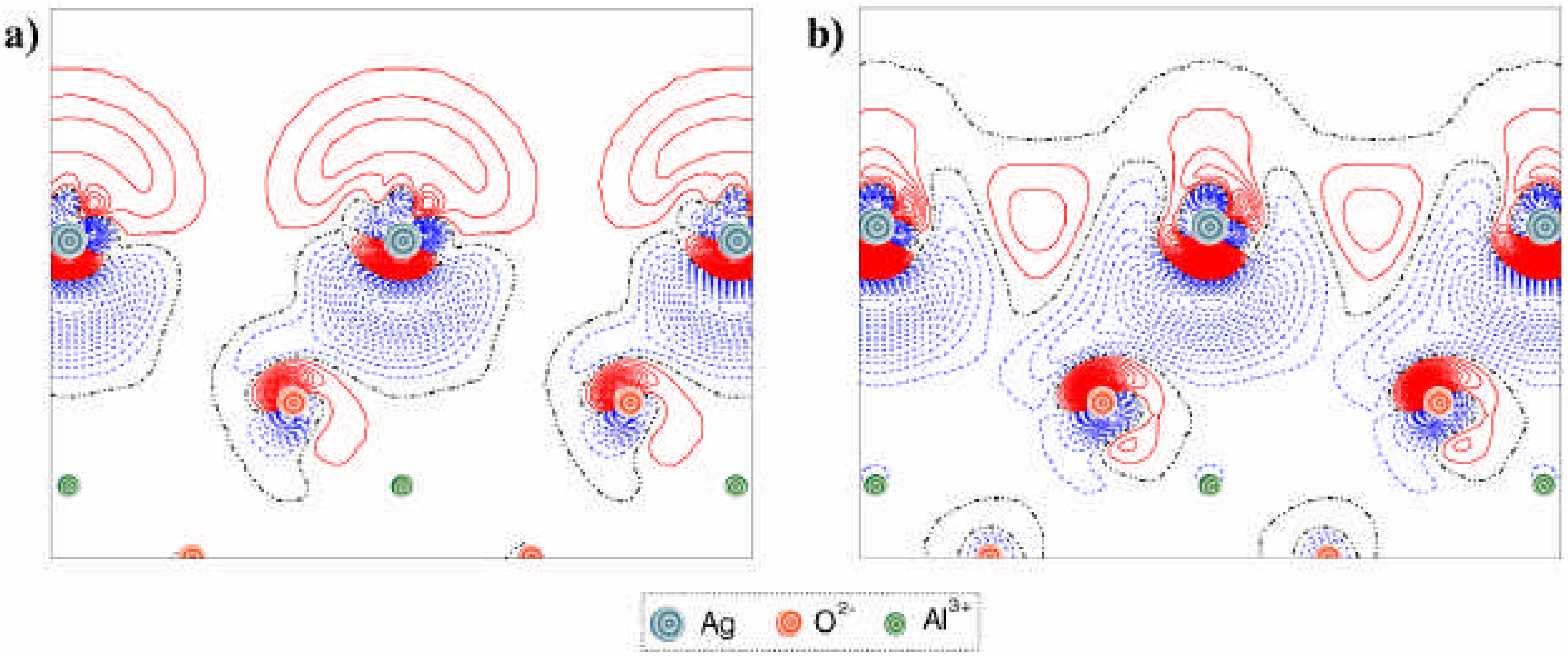
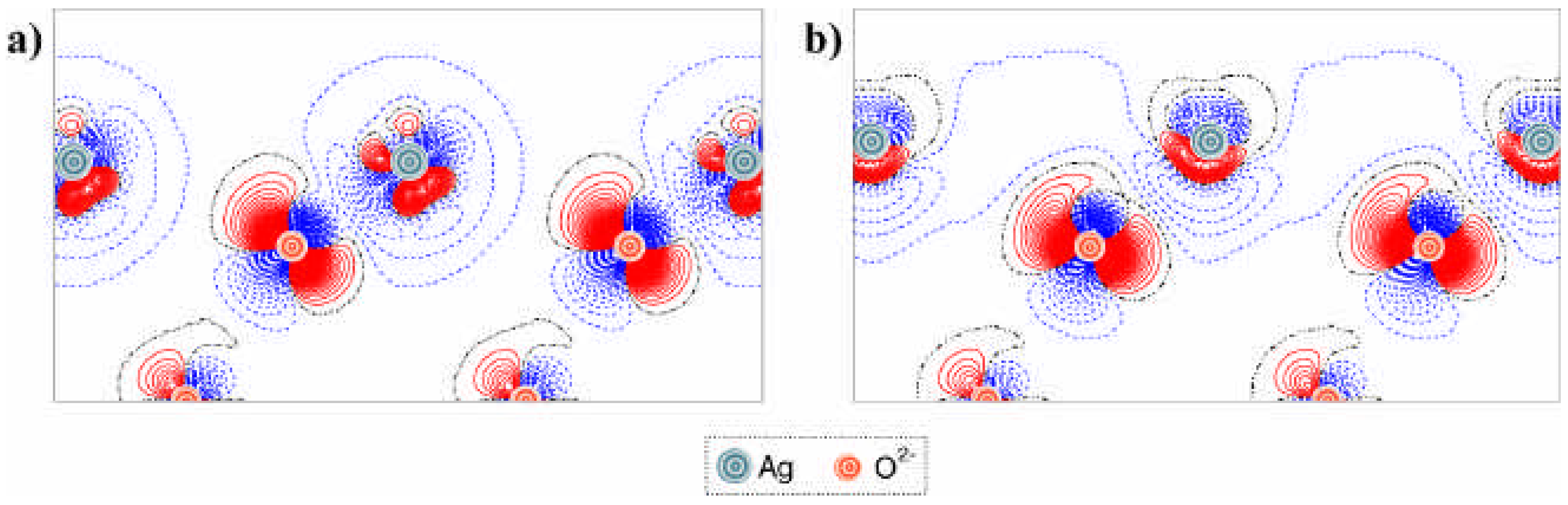
ΔqAg, characterizes the interfacial electronic charge transfer (
Table 1). To obtain a greater insight into the nature of the interaction between the corundum surface and quasi-isolated silver adatoms (1/3 ML) or the full (111) monolayer, we have plotted several 2D sections of the
difference electron density distribution
Δρ(
r) across the interface (
Fig. 4 and
Fig. 5). This charge redistribution function is defined as:
i.e. the total electron density
ρ Ag / corundum slab(
r) minus a superposition of the densities for the two isolated metal and corundum slabs,
ρ Ag slab(
r) and
ρ corundum slab(
r), respectively, with the same geometry as they have in the interface system. Each density plot can clearly demonstrate the effects resulting from the interfacial interactions. The projections for the cross-sections used for these plots are defined in
Fig. 3.