Transforming Growth Factor-β3/Chitosan Sponge (TGF-β3/CS) Facilitates Osteogenic Differentiation of Human Periodontal Ligament Stem Cells
Abstract
:1. Introduction
2. Results
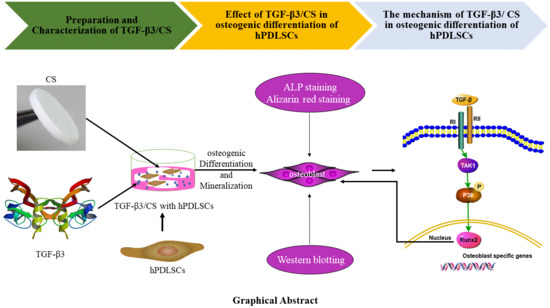
2.1. Preparation and Characterization of Transforming Growth Factor-β3/Chitosan Sponge (TGF-β3/CS)
2.2. TGF-β3 Does not Affect Growth or Proliferation of hPDLSCs But Facilitates Their Osteogenic Differentiation
2.3. TGF-β3/CS Facilitates Osteogenic Differentiation of hPDLSCs
2.4. TGF-β3 Promotes Osteogenic Differentiation of hPDLSCs via the p38 MAPK Pathway
3. Discussion
4. Materials and Methods
4.1. Preparation and Characterization of TGF-β3/CS
Release Profile of TGF-β3 from CS
4.2. Culture of hPDLSCs
4.3. Bioactivity and Biocompatibility Assays in Vitro
4.3.1. Biocompatibility Analysis of TGF-β3/CS Extract
4.3.2. Cell Proliferation Assay of hPDLSCs Loaded on TGF-β3/CS in Vitro
4.3.3. Growth of hPDLSCs Implanted in TGF-β3/CS in Vitro
4.4. Growth of hPDLSCs Implanted in TGF-β3/CS in Vivo
4.5. Induction of Osteogenic Differentiation in Vitro
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goker, F.; Larsson, L.; Del Fabbro, M.; Asa’ad, F. Gene delivery therapeutics in the treatment of periodontitis and peri-implantitis: A state of the art review. Int. J. Mol. Sci. 2019, 20, 3551. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.; Pandis, N.; Mustafa, K.; Nyengaard, J.R.; Stavropoulos, A. Alveolar bone tissue engineering in critical-size defects of experimental animal models: A systematic review and meta-analysis. J. Tissue Eng. Regen. Med. 2017, 11, 2935–2949. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Decker, A.M.; Nibali, L.; Pilipchuk, S.P.; Berglundh, T.; Giannobile, W.V. Regenerative medicine for periodontal and peri-implant diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Al-Askar, M.; Alsaffar, D. Feasibility of using allograft bone with resorbable collagen membrane for alveolar ridge vertical defect augmentation for dental implant placement in patient with aggressive periodontitis: A case report. Saudi Dent. J. 2018, 30, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Akintoye, S.O. The distinctive jaw and alveolar bone regeneration. Oral Dis. 2018, 24, 49–51. [Google Scholar] [CrossRef] [PubMed]
- EzEldeen, M.; Wyatt, J.; Al-Rimawi, A.; Coucke, W.; Shaheen, E.; Lambrichts, I.; Willems, G.; Politis, C.; Jacobs, R. Use of cbct guidance for tooth autotransplantation in children. J. Dent. Res. 2019, 98, 406–413. [Google Scholar] [CrossRef]
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A.; et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res. Ther. 2018, 9, 213. [Google Scholar] [CrossRef]
- Kloss, F.R.; Offermanns, V.; Kloss-Brandstatter, A. Cecomparison of allogeneic and autogenous bone grafts for augmentation of alveolar ridge defects—A 12-month retrospective radiographic evaluation. Clin. Oral Implant. Res. 2018, 29, 1163–1175. [Google Scholar] [CrossRef]
- Hameed, M.H.; Gul, M.; Ghafoor, R.; Khan, F.R. Vertical ridge gain with various bone augmentation techniques: A systematic review and meta-analysis. J. Prosthodont. 2019, 28, 421–427. [Google Scholar] [CrossRef]
- Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, current strategies, and novel nano-technological approaches for periodontal regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Montero, E.; Monje, A.; Sanz-Sanchez, I. Effectiveness of vertical ridge augmentation interventions. A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.A.; Kao, R.T.; Camargo, P.M.; Caton, J.G.; Clem, D.S.; Fiorellini, J.P.; Geisinger, M.L.; Mills, M.P.; Nares, S.; Nevins, M.L. Periodontal regeneration—Intrabony defects: A consensus report from the aap regeneration workshop. J. Periodontol. 2015, 86, S105–S107. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of gtr/gbr membranes for periodontal regeneration—A materials perspective. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [PubMed]
- Sasaki, H.; Rothrauff, B.B.; Alexander, P.G.; Lin, H.; Gottardi, R.; Fu, F.H.; Tuan, R.S. In vitro repair of meniscal radial tear with hydrogels seeded with adipose stem cells and tgf-beta3. Am. J. Sports Med. 2018, 46, 2402–2413. [Google Scholar] [CrossRef]
- Lee, H.L.; Yu, B.; Deng, P.; Wang, C.Y.; Hong, C. Transforming growth factor-beta-induced kdm4b promotes chondrogenic differentiation of human mesenchymal stem cells. Stem Cells 2016, 34, 711–719. [Google Scholar] [CrossRef]
- Yang, Q.; Teng, B.H.; Wang, L.N.; Li, K.; Xu, C.; Ma, X.L.; Zhang, Y.; Kong, D.L.; Wang, L.Y.; Zhao, Y.H. Silk fibroin/cartilage extracellular matrix scaffolds with sequential delivery of tgf-beta3 for chondrogenic differentiation of adipose-derived stem cells. Int. J. Nanomed. 2017, 12, 6721–6733. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Zhang, X.; Gao, M.; Luo, K.; Fu, W.; Yin, M.; Wang, W.; Zhu, Z.; Zheng, J.; He, X. Kartogenin preconditioning commits mesenchymal stem cells to a precartilaginous stage with enhanced chondrogenic potential by modulating jnk and beta-catenin-related pathways. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 5641–5653. [Google Scholar]
- Grafe, I.; Alexander, S.; Peterson, J.R.; Snider, T.N.; Levi, B.; Lee, B.; Mishina, Y. Tgf-beta family signaling in mesenchymal differentiation. Cold Spring Harb. Perspect. Biol. 2018, 10, a022202. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Mei, T.; Hou, T.; Luo, K.; Luo, F.; Yang, A.; Yu, B.; Pang, H.; Dong, S.; Xu, J. Tgfbeta3 recruits endogenous mesenchymal stem cells to initiate bone regeneration. Stem Cell Res. Ther. 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Dix-Peek, T.; Parak, R.; Milner, B.; Duarte, R. Profiling bone morphogenetic proteins and transforming growth factor-betas by htgf-beta3 pre-treated coral-derived macroporous bioreactors: The power of one. Biomaterials 2015, 49, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U. Developmental pathways of periodontal tissue regeneration: Developmental diversities of tooth morphogenesis do also map capacity of periodontal tissue regeneration? J. Periodontal Res. 2019, 54, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Klar, R.M.; Duarte, R.; Dix-Peek, T.; Ripamonti, U. The induction of bone formation by the recombinant human transforming growth factor-beta3. Biomaterials 2014, 35, 2773–2788. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Ramoshebi, L.N.; Teare, J.; Renton, L.; Ferretti, C. The induction of endochondral bone formation by transforming growth factor-3: Experimental studies in the non-human primate papio ursinus. J. Cell. Mol. Med. 2008, 12, 1029–1048. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Duarte, R.; Ferretti, C. Re-evaluating the induction of bone formation in primates. Biomaterials 2014, 35, 9407–9422. [Google Scholar] [CrossRef]
- Moioli, E.K.; Clark, P.A.; Sumner, D.R.; Mao, J.J. Autologous stem cell regeneration in craniosynostosis. Bone 2008, 42, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Rattan, V.; Rai, S. Efficacy of chitosan in promoting wound healing in extraction socket: A prospective study. J. Oral Biol. Craniofac. Res. 2019, 9, 91–95. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, F.; Zhang, B.; Zhou, M.; Bei, Y.; Zhang, Y.; Tang, J.; Yang, Y.; Huang, Y.; Xiang, Q.; et al. Improving the protective effects of afgf for peripheral nerve injury repair using sulfated chitooligosaccharides. Asian J. Pharm. Sci. 2019, 14, 511–520. [Google Scholar] [CrossRef]
- Safari, S.; Mahdian, A.; Motamedian, S.R. Applications of stem cells in orthodontics and dentofacial orthopedics: Current trends and future perspectives. World J. Stem Cells 2018, 10, 66–77. [Google Scholar] [CrossRef]
- Abdel Meguid, E.; Ke, Y.; Ji, J.; El-Hashash, A.H.K. Stem cells applications in bone and tooth repair and regeneration: New insights, tools, and hopes. J. Cell. Physiol. 2018, 233, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, K.H.; Seo, B.M.; Koo, K.T.; Kim, T.I.; Seol, Y.J.; Ku, Y.; Rhyu, I.C.; Chung, C.P.; Lee, Y.M. Alveolar bone regeneration by transplantation of periodontal ligament stem cells and bone marrow stem cells in a canine peri-implant defect model: A pilot study. J. Periodontol. 2009, 80, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Liu, H.; Liang, Q.; Shang, L.; Wang, T.; Ge, S. Oxytocin facilitates the proliferation, migration and osteogenic differentiation of human periodontal stem cells in vitro. Arch. Oral Biol. 2019, 99, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Q.; Zhou, Y.; Li, W. Tlr activation inhibits the osteogenic potential of human periodontal ligament stem cells through akt signaling in a myd88-or trif-dependent manner. J. Periodontol. 2019, 90, 400–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, F.; Song, Y.; Duan, Y.; Jin, Z. Erythropoietin induces the osteogenesis of periodontal mesenchymal stem cells from healthy and periodontitis sources via activation of the p38 mapk pathway. Int. J. Mol. Med. 2018, 41, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. Tgf-beta and bmp signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang, Y.J.; Dong, G.Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef]
- Feldman, D.S.; McCauley, J.F. Mesenchymal stem cells and transforming growth factor-beta(3) (tgf-beta(3)) to enhance the regenerative ability of an albumin scaffold in full thickness wound healing. J. Funct. Biomater. 2018, 9, 65. [Google Scholar] [CrossRef]
- Menon, A.H.; Soundarya, S.P.; Sanjay, V.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Sustained release of chrysin from chitosan-based scaffolds promotes mesenchymal stem cell proliferation and osteoblast differentiation. Carbohydr. Polym. 2018, 195, 356–367. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, D.; Yu, S.; Fu, J.; Li, Z.; Wu, Y.; Wang, Y. Sall4 promotes osteoblast differentiation by deactivating notch2 signaling. Biomed. Pharmacother. 2018, 98, 9–17. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chang, Y.H.; Li, K.C.; Lu, C.H.; Sung, L.Y.; Yeh, C.L.; Lin, K.J.; Huang, S.F.; Yen, T.C.; Hu, Y.C. The use of ascs engineered to express bmp2 or tgf-beta3 within scaffold constructs to promote calvarial bone repair. Biomaterials 2013, 34, 9401–9412. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.M.; Herberg, S.; Mason, D.E.; Collins, J.M.; Pearson, H.B.; Dawahare, J.H.; Tang, R.; Patwa, A.N.; Grinstaff, M.W.; Kelly, D.J.; et al. Recapitulating bone development through engineered mesenchymal condensations and mechanical cues for tissue regeneration. Sci. Transl. Med. 2019, 11, eaav7756. [Google Scholar] [CrossRef] [PubMed]
- Aisenbrey, E.A.; Bryant, S.J. The role of chondroitin sulfate in regulating hypertrophy during msc chondrogenesis in a cartilage mimetic hydrogel under dynamic loading. Biomaterials 2019, 190-191, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.; Pigeot, S.; Muller, J.; Schaefer, D.J.; Martin, I.; Scherberich, A. Fractionated human adipose tissue as a native biomaterial for the generation of a bone organ by endochondral ossification. Acta Biomater. 2018, 77, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Paolella, F.; Gabusi, E.; Manferdini, C.; Schiavinato, A.; Lisignoli, G. Specific concentration of hyaluronan amide derivative induces osteogenic mineralization of human mesenchymal stromal cells: Evidence of runx2 and col1a1 genes modulation. J. Biomed. Mater. Res. Part A 2019. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.E.L.; Vandrovcova, M.; Krocilova, N.; Keppler, J.K.; Zarubova, J.; Skirtach, A.G.; Bacakova, L. Application of whey protein isolate in bone regeneration: Effects on growth and osteogenic differentiation of bone-forming cells. J. Dairy Sci. 2018, 101, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, S.; Dong, G.; Peng, B.; Xu, J.; Ma, Z.; Wang, X.; Liu, L.; Wang, Q. A comparison of physicochemical properties of sterilized chitosan hydrogel and its applicability in a canine model of periodontal regeneration. Carbohydr. Polym. 2014, 113, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Laidmae, I.; Erglis, K.; Cebers, A.; Janmey, P.A.; Uibo, R. Salmon fibrinogen and chitosan scaffold for tissue engineering: In vitro and in vivo evaluation. J. Mater. Sci. Mater. Med. 2018, 29, 182. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, S.; Hiroaki, T.; Masahide, T.; Kenichiro, M.; Tomomi, N.; Keigo, S.; Mariko, K.; Toshihito, A.; Hiroyuki, O.; Takanobu, K. Fibroblast growth factor-2 stimulates directed migration of periodontal ligament cells via pi3k/akt signaling and cd44/hyaluronan interaction. J. Cell. Physiol. 2011, 226, 809–821. [Google Scholar]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Qiao, Z.; Yu, F.; Hu, H.; Huang, Y.; Xiang, Q.; Zhang, Q.; Yang, Y.; Zhao, Y. Transforming Growth Factor-β3/Chitosan Sponge (TGF-β3/CS) Facilitates Osteogenic Differentiation of Human Periodontal Ligament Stem Cells. Int. J. Mol. Sci. 2019, 20, 4982. https://doi.org/10.3390/ijms20204982
Li Y, Qiao Z, Yu F, Hu H, Huang Y, Xiang Q, Zhang Q, Yang Y, Zhao Y. Transforming Growth Factor-β3/Chitosan Sponge (TGF-β3/CS) Facilitates Osteogenic Differentiation of Human Periodontal Ligament Stem Cells. International Journal of Molecular Sciences. 2019; 20(20):4982. https://doi.org/10.3390/ijms20204982
Chicago/Turabian StyleLi, Yangfan, Zhifen Qiao, Fenglin Yu, Huiting Hu, Yadong Huang, Qi Xiang, Qihao Zhang, Yan Yang, and Yueping Zhao. 2019. "Transforming Growth Factor-β3/Chitosan Sponge (TGF-β3/CS) Facilitates Osteogenic Differentiation of Human Periodontal Ligament Stem Cells" International Journal of Molecular Sciences 20, no. 20: 4982. https://doi.org/10.3390/ijms20204982