Methyl Parathion Masks Withdrawal from Physical Dependence on Morphine
Abstract
:Introduction
2. Materials and methods
2.1. Animals
2.2. Induction of morphine dependence
2.3. Pretreatment with MP
2.4. Behavioral assessment
2.5. Brain cholinesterase assay
2.6. Statistics
3. Results
3.1. MP-induced toxic signs and suppression of spontaneous locomotor activity
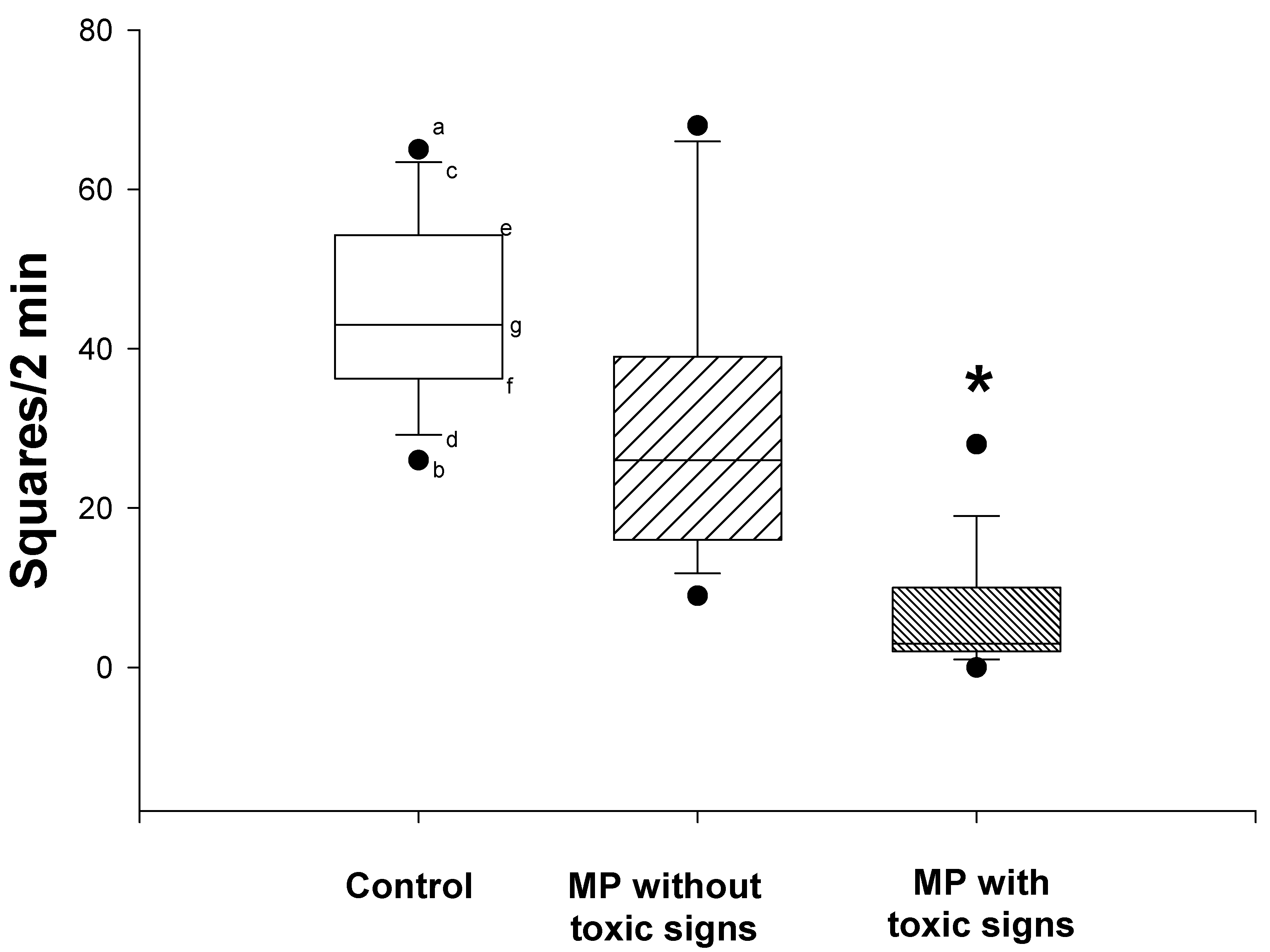
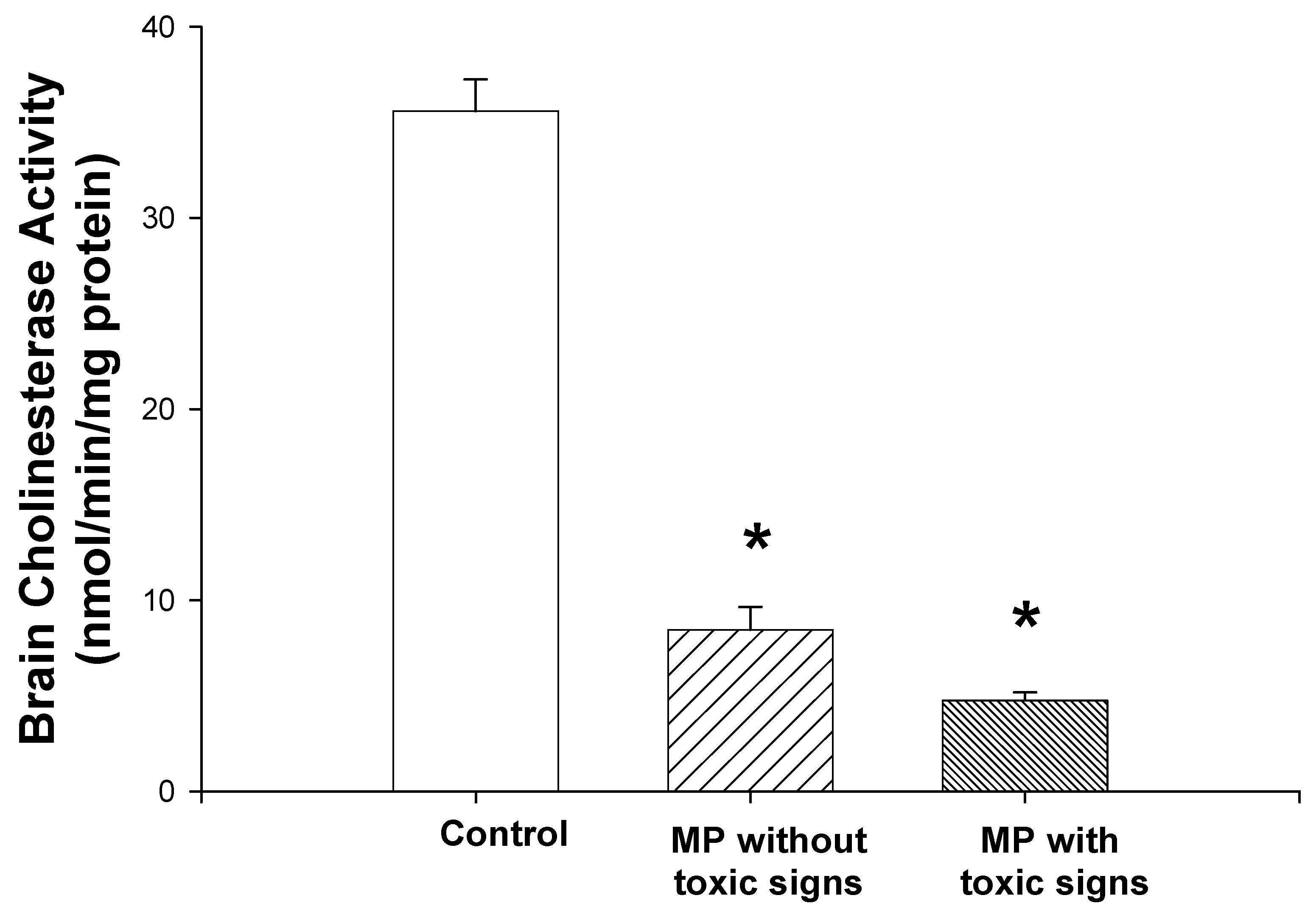
3.2. Brain cholinesterase activity
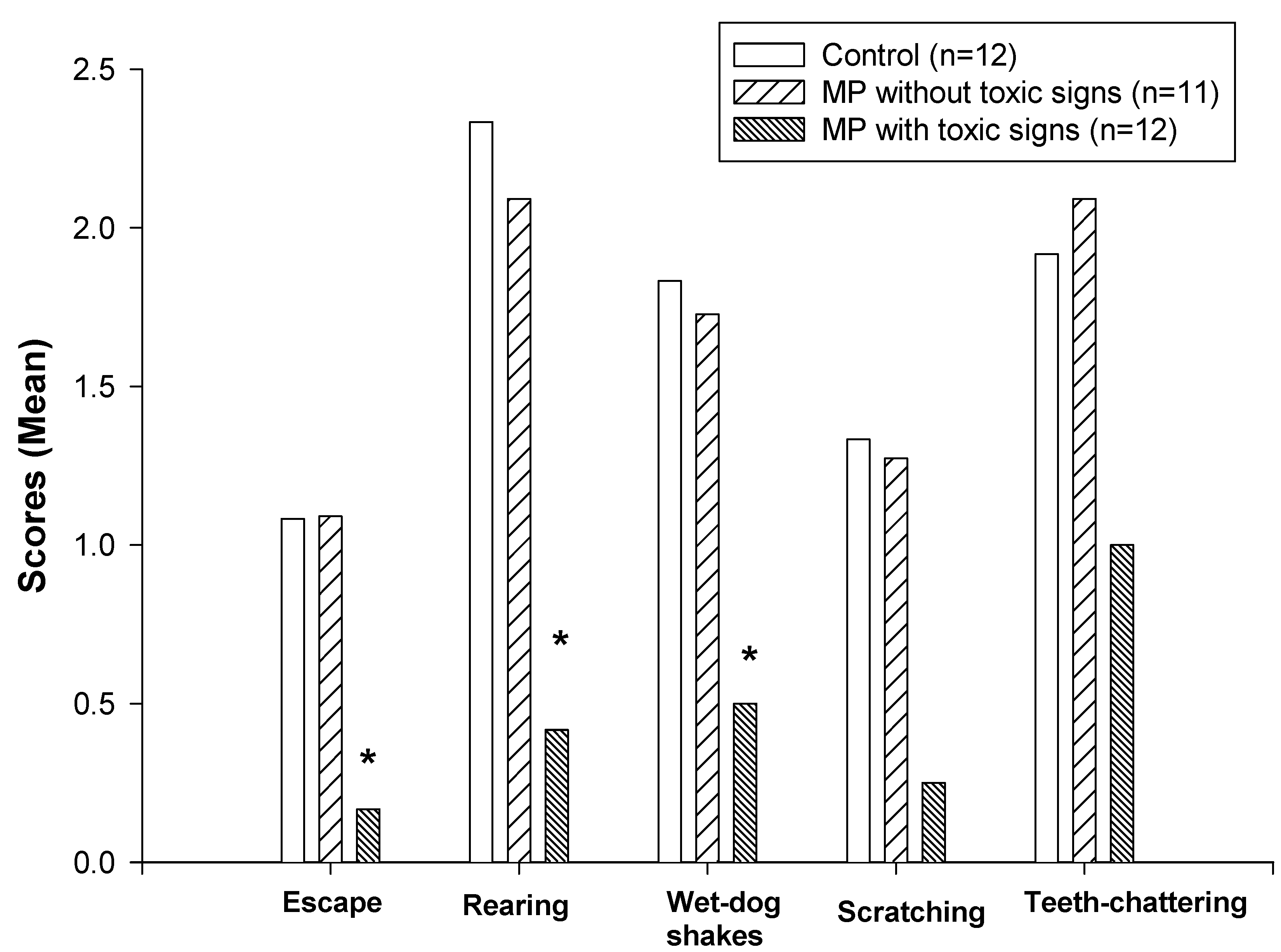
3.3. Naloxone-precipitated withdrawal from morphine
4. Discussion
| Control (N = 12) | MP with Toxic Signs (N = 12) | MP without Toxic Signs (N = 11) | |
|---|---|---|---|
| Vocalization | 5/12 | 6/12 | 4/11 |
| Penis licking | 7/12 | 7/12 | 5/11 |
| Ptosis | 10/12 | 11/12 | 9/11 |
Acknowledgements
References and Notes
- Costa, E.; Cheney, D.L.; Racagni, G.; Zsilla, G. An analysis at synaptic level of the morphine action in striatum and N. accumbens: dopamine and acetylcholine interactions. Life Sci. 1975, 17(1), 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moroni, F.; Cheney, D.L.; Costa, E. The turnover rate of acetylcholine in brain nuclei of rats injected intraventricularly and intraseptally with alpha and beta-endorphin. Neuropharmacology 1978, 17(3), 191–61. [Google Scholar] [CrossRef] [PubMed]
- Mulder, A.H.; Wardeh, G.; Hogenboom, F.; Frankhuyzen, A.L. Kappa- and delta-opioid receptor agonists differentially inhibit striatal dopamine and acetylcholine release. Nature 1984, 308(5956), 278–80. [Google Scholar] [CrossRef] [PubMed]
- Ennis, C.; Wyllie, M.G. Evidence for functionally distinct mu receptors modulating acetylcholine release. Neuropeptides 1984, 5(1-3), 109–12. [Google Scholar] [CrossRef] [PubMed]
- Lapchak, P.A.; Araujo, D. M.; Collier, B. Regulation of endogenous acetylcholine release from mammalian brain slices by opiate receptors: hippocampus, striatum and cerebral cortex of guinea-pig and rat. Neuroscience 1989, 31(2), 313–25. [Google Scholar] [CrossRef] [PubMed]
- Domino, E.F.; Wilson, A.E. Letter: Enhanced utilization of brain acetylcholine during morphine withdrawal in the rat. Nature 1973, 243(5405), 285–6. [Google Scholar] [CrossRef] [PubMed]
- Casamenti, F.; Pedata, F.; Corradetti, R.; Pepeu, G. Acetylcholine input from the cerebral cortex, choline uptake and muscarinic receptors in morphine-dependent, freely-moving rats. Neuropharmacology 1980, 19(7), 597–605. [Google Scholar] [CrossRef] [PubMed]
- Redmond, D.E.; Krystal, J.H. Multiple mechanisms of withdrawal from opioid drugs. Ann. Rev. Neurosci. 1984, 7, 443–78. [Google Scholar] [CrossRef] [PubMed]
- Buccafusco, J.J. Neuropharmacologic and behavioral actions of clonidine: interactions with central neurotransmitters. Intern. Rev. Neurobiol. 1992, 33, 55–107. [Google Scholar]
- Buccafusco, J.J.; Zhang, L.C.; Shuster, L.C.; Jonnala, R.R.; Gattu, M. Prevention of precipitated withdrawal symptoms by activating central cholinergic systems during a dependence-producing schedule of morphine in rats. Brain Res. 2000, 852(1), 76–83. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P. Anticholinesterase agents. In Goodman and Gillman’s The Pharmacological Basis of TherapeuticsHardman, J.G., Limbird, L.E., Molinoff, P.B., Ruddon, R.W., Gilman, A.G., Eds.; McGraw-Hill: New York, 9th ed.; 1996; chapter 8. [Google Scholar]
- Zhu, H.; Rockhold, R.W.; Baker, R.C.; Kramer, R.E.; Ho, I.K. Effects of repeated or single dermal administration of methyl parathion on behavior and cholinesterase activity in rats. J. Biomed. Sci. 2001, 8, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic CoordinatesAcademic Press: Orlando, 2nd ed.; 1986. [Google Scholar]
- Zhu, H.; Ho, I.K. NMDA-R1 antisense oligonucleotide attenuates withdrawal signs from morphine. Eur. J. Pharm. 1998, 352(2-3), 151–6. [Google Scholar] [CrossRef]
- Nostrandt, A. C.; Duncan, J.A.; Padilla, S. A modified spectrophotometric method appropriate for measuring cholinesterase activity in tissue from carbaryl-treated animals. Fund. Appl. Toxicol. 1993, 21(2), 196–203. [Google Scholar] [CrossRef]
- Ellman, G.C.; Courtney, K.O.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–54. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.C.; Buccafusco, J.J. Spinal cholinergic neurons and the expression of morphine withdrawal symptoms in the rat. J. Neurosci. 1987, 7(3), 621–8. [Google Scholar] [PubMed] [Green Version]
© 2002 by MDPI (http://www.mdpi.org).
Share and Cite
Zhu, H.; Ho, I.K.; Kramer, R.E.; Baker, R.C.; Rockhold, R.W. Methyl Parathion Masks Withdrawal from Physical Dependence on Morphine. Int. J. Mol. Sci. 2002, 3, 1073-1081. https://doi.org/10.3390/i3101073
Zhu H, Ho IK, Kramer RE, Baker RC, Rockhold RW. Methyl Parathion Masks Withdrawal from Physical Dependence on Morphine. International Journal of Molecular Sciences. 2002; 3(10):1073-1081. https://doi.org/10.3390/i3101073
Chicago/Turabian StyleZhu, Hong, Ing K. Ho, Robert E. Kramer, Rodney C. Baker, and Robin W. Rockhold. 2002. "Methyl Parathion Masks Withdrawal from Physical Dependence on Morphine" International Journal of Molecular Sciences 3, no. 10: 1073-1081. https://doi.org/10.3390/i3101073




