Cationic Polymerization of 1,2-Epoxypropane by an Acid Exchanged Montmorillonite Clay in the Presence of Ethylene Glycol
Abstract
:Introduction
Experimental
Materials
Characterization
Results and Discussion
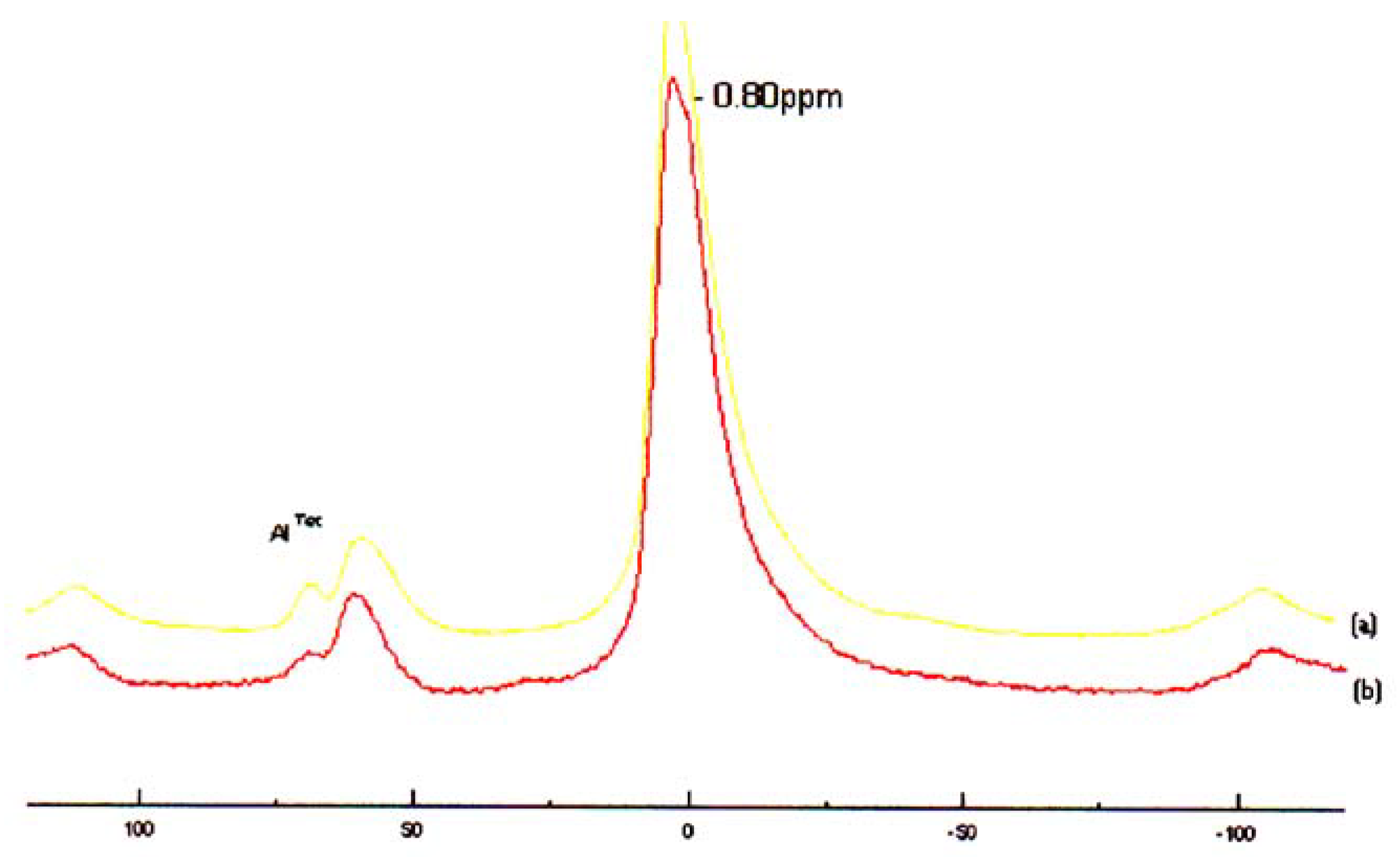
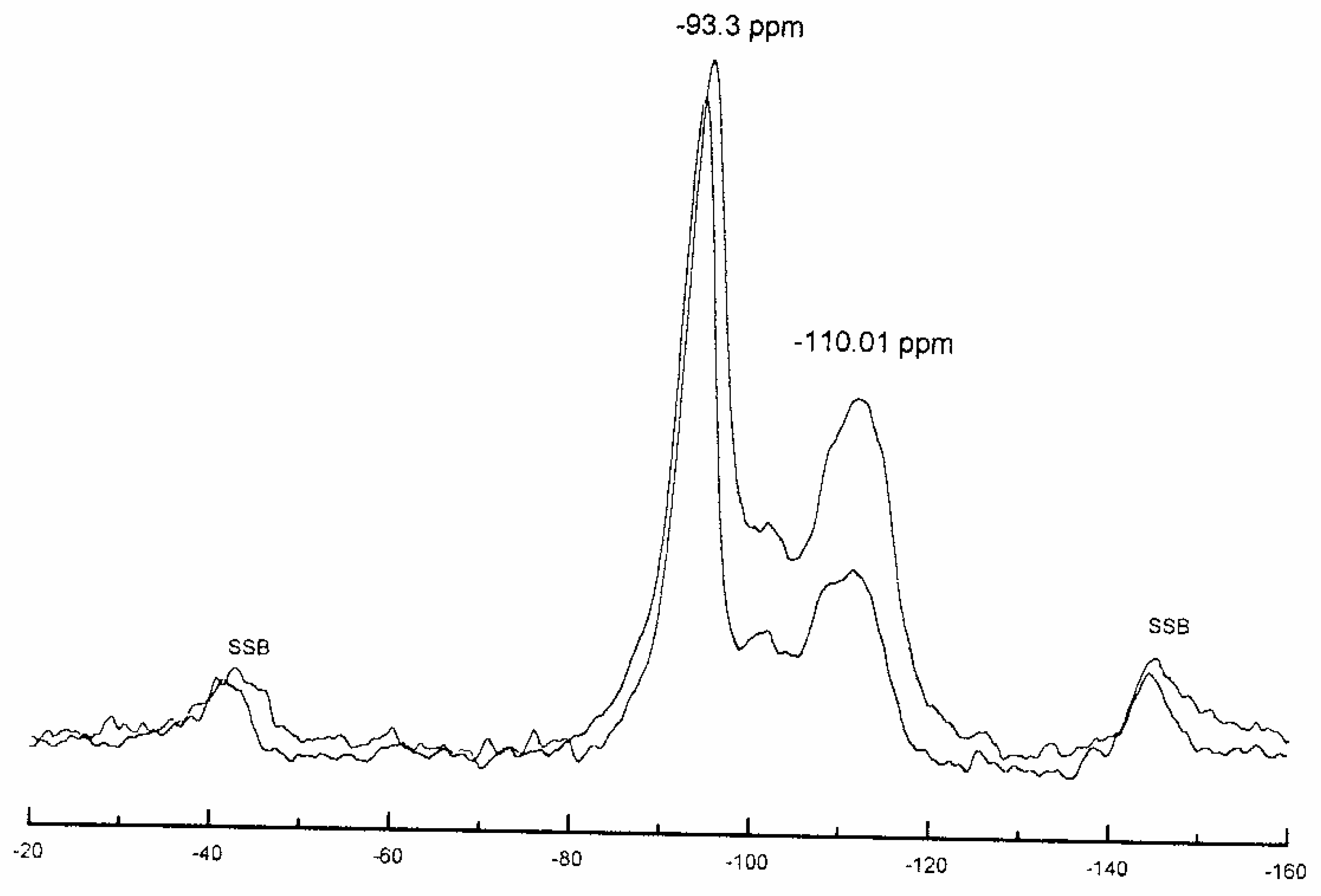
Catalyst Structure
| Composition wt% | |||||||||||
| Sample | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | SO3 | As | PF* |
| Raw-Maghnite | 69.39 | 14.67 | 1.16 | 0.3 | 1.07 | 0.5 | 0.79 | 0.16 | 0.91 | 0.05 | 11 |
| H-Mag0.05M | 70.75 | 14.67 | 1.05 | 0.3 | 1.01 | 0.49 | 0.78 | 0.16 | 0.75 | 0.04 | 10 |
| H-Mag0.10M | 71 | 14.6 | 1 | 0.3 | 0.98 | 0.39 | 0.78 | 0.16 | 0.55 | 0.04 | 10 |
| H-Mag0.15M | 71.58 | 14.45 | 0.95 | 0.29 | 0.91 | 0.35 | 0.77 | 0.15 | 0.42 | 0.03 | 10 |
| H-Mag0.20M | 71.65 | 14.2 | 0.8 | 0.28 | 0.85 | 0.3 | 0.77 | 0.15 | 0.39 | 0.01 | 10 |
| H-Mag0.25M | 71.7 | 14.03 | 0.71 | 0.28 | 0.8 | 0.21 | 0.77 | 0.15 | 0.34 | 0.01 | 11 |
| H-Mag0.30M | 73.2 | 13.85 | 0.7 | 0.27 | 0.78 | 0.2 | 0.76 | 0.13 | 0.31 | 0.02 | 9.78 |
| H-Mag0.35M | 75.31 | 13.52 | 0.71 | 0.26 | 0.78 | 0.18 | 0.75 | 0.13 | 0.32 | 0.01 | 8.03 |
| Wyoming (USA)[25] | Vienne (France)[26] | Raw-Maghnite (Algeria) | H-Maghnite (Algeria) | |
| SiO2 | 50.04 | 57.49 | 69.39 | 71.70 |
| Al2O3 | 20.16 | 20.27 | 14.67 | 14.03 |
| Fe2O3 | 0.68 | 2.92 | 1.16 | 0.71 |
| FeO | 0.19 | |||
| CaO | 1.46 | 0.23 | 0.30 | 0.28 |
| MgO | 0.23 | 3.13 | 1.07 | 0.80 |
| K2O | 1.27 | 0.28 | 0.79 | 0.77 |
| Na2O | Tr | 1.32 | 0.5 | 0.21 |
| TiO2 | 0.12 | 0.16 | 0.15 | |
| SO3 | 0.91 | 0.34 | ||
| As | 0.05 | 0.01 |

| Samples | dhkl (A°) | hkl | Nature of sample |
| Raw-Maghnite | 12.50 | 001 | Montmorillonite |
| 4.47 | 110 | Montmorillonite | |
| 4.16 | ,, | Quartz | |
| 3.35 | ,, | Quartz | |
| 3.21 | ,, | Feldspath | |
| 3.03 | ,, | Calcite | |
| 2.55 | 200 | Montmorillonite | |
| 1.68 | 009 | Montmorillonite | |
| 1.49 | 060 | Montmorillonite | |
| Mag-H+0.25M | 15.02 | 001 | Montmorillonite |
| 4.47 | 110 | Montmorillonite | |
| 4.16 | ,, | Quartz | |
| 3.35 | ,, | Quartz | |
| 3.21 | ,, | Feldspath | |
| 3.03 | ,, | Calcite | |
| 2.55 | 200 | Montmorillonite | |
| 1.68 | 009 | Montmorillonite | |
| 1.49 | 060 | Montmorillonite |

Polymerization and Products Characterization
| Experiment | PO (g) | [PO] | “H-Maghnite0.25M”(g) | Yield % | Mv | Mn | Mw | I=Mw/Mn |
| 1 (Bulk) | 10 | - | 0.1 | 15 | 520 | 349 | 771 | 2.21 |
| 2 (Bulk) | 10 | - | 0.05 | 04 | 910 | 746 | 993 | 1.33 |
| 3 (CH2Cl2) | 5 | 5 | 0.1 | 11 | 896 | 610 | 1050 | 1.72 |
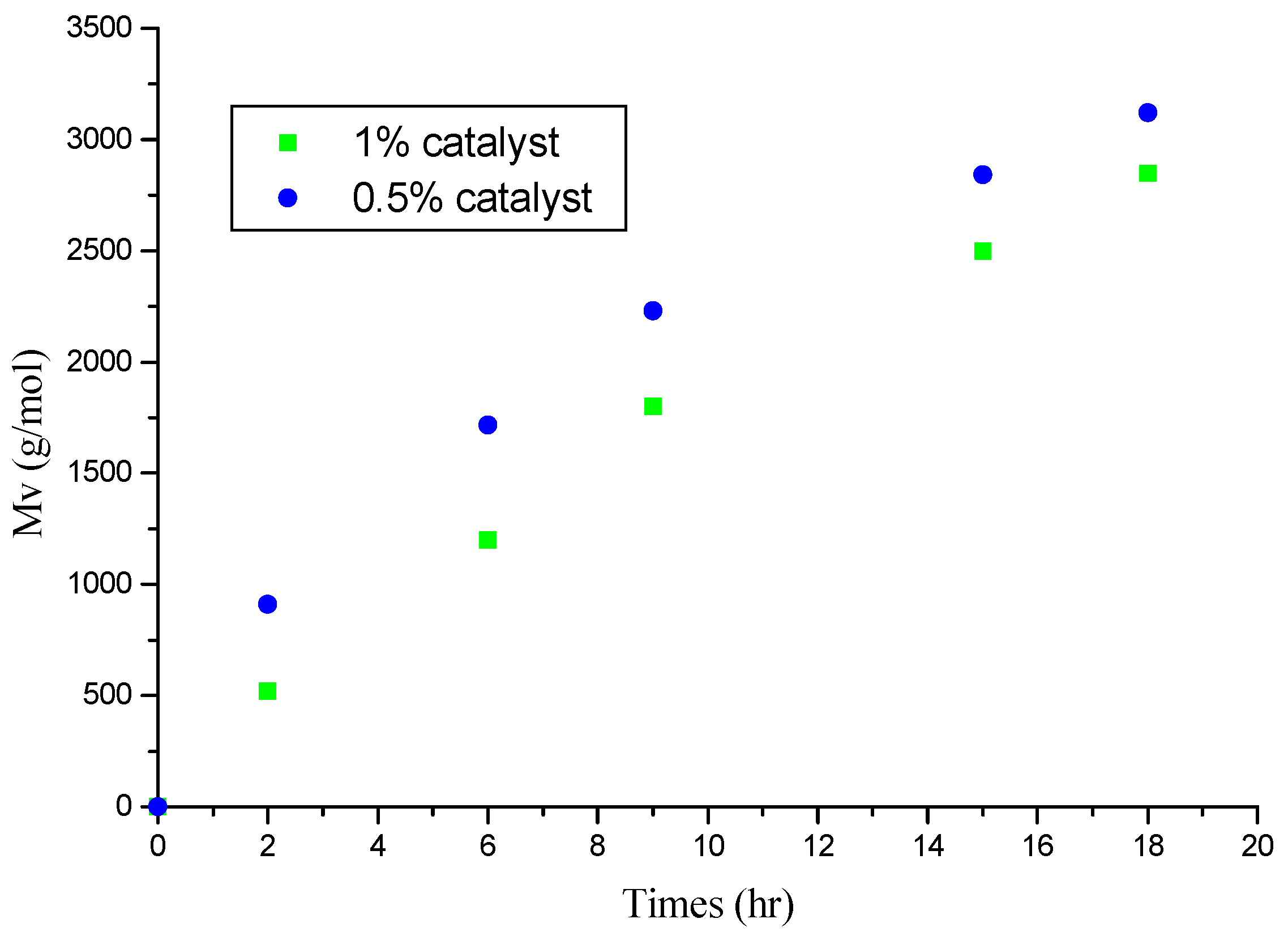
Effect of Mag-H+ 0.25M Proportion
| Time(h) | 2 | 6 | 9 | 15 | 18 | 20 | 22 | 26 | 30 |
| Yield(%)(a) | 15 | 21 | 30 | 42 | 52 | 60 | 67 | 77 | 80 |
| Yield(%)(b) | 4 | 15 | 26 | 30 | 35 | 39 | 50 | 52 | 54 |

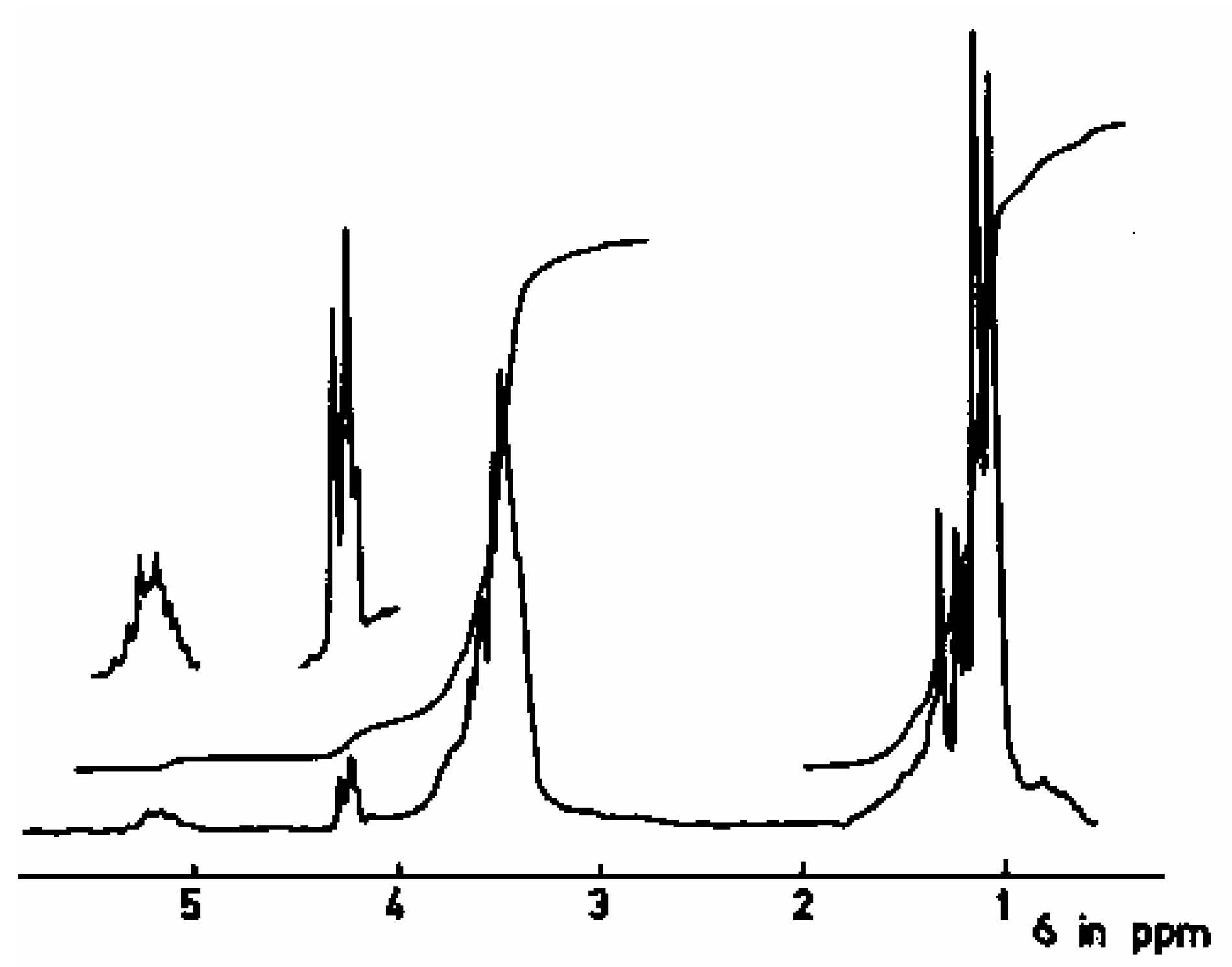
1 H-NMR Study
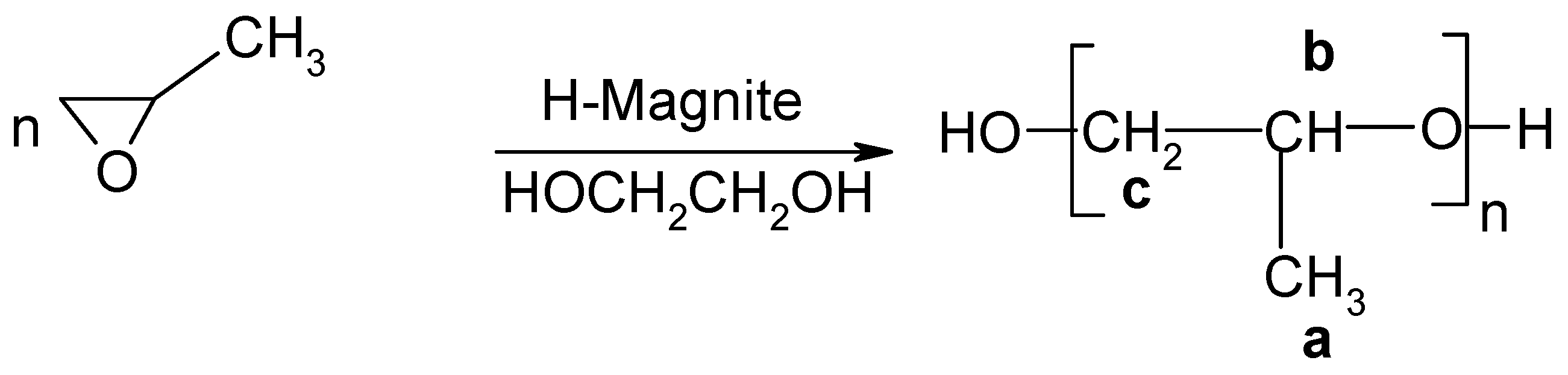
| Proton type | (a) | (b) | (c) |
| δ(ppm) | 1.1 | 3.5 | 3.5 |

End-Groups


Effect of Solvent
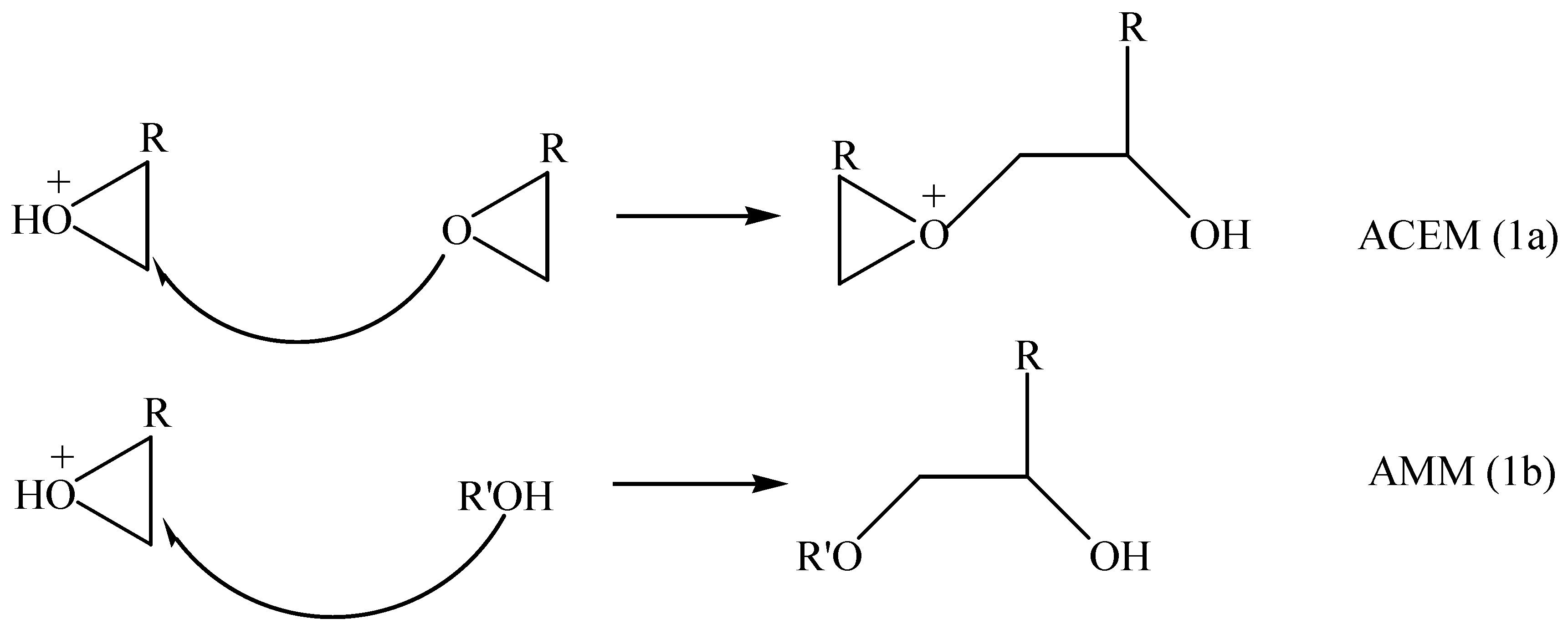
Polymerization Mechanism
Initiation

Propagation


Conclusion
Acknowledgements
References
- Mueller, H.; Huchler, O. H; Hoffmann, H. US Patent 4 243 799, 1981.
- Farbenfabriken Bayer. German Patent 1 049 103, 1959.
- Fujita, K.; Ishida, Y; Suezana, J. Japan Kokai Tokkyo Toho. JP 62 289 537, 1987. [Google Scholar]
- Vu Moc, T.; Petit, H; Maitte, P. Bull. Soc. Belg 1982, 91, 261.
- Schelly, J. S.; Mather, P. T; DeVries, K. L. Polymer 2001, 42, 5849. [CrossRef]
- Vaia, R. A.; Jandt, K. D.; Kramer, E. J; Giannelis, E. P. Macromolecules 1995, 28, 8080. [CrossRef]
- Vaia, R. A; Giannelis, E. P. Macromolecules 1997, 30, 7990. [CrossRef]
- Yoon, J. T.; Jo, W. H.; Lee, M. S; Ko, M. B. Polymer 2001, 42, 329. [CrossRef]
- Lee, J.; Park, M. S.; Yang, H. C.; Cho, K; Kim, J. K. Polymer 2003, 44, 1705. [CrossRef]
- Dreyfuss, M. P. J. Macromol. Sci. Chem. 1975, A9, 729. [CrossRef]
- Kern, R. J. J.Org. Chem. 1968, 33, 388.
- Libszowski, J.; Szymanski, R; Penczek, S. Makromol.Chem. 1989, 190, 1225. [CrossRef]
- Biedron, T.; Kubisa, P; Penczek, S. J. Polym. Sci.: Pt A: Polym Chem. 1991, 29, 619. [CrossRef]
- Tokar, R.; Kubisa, P.; Penczek, S; Dworak, A. Macromolecules 1994, 27, 320. [CrossRef]
- Bednarek, M.; Kubisa, P; Penczek, S. Makromol.Chem.Suppl . 1989, 15, 49. [CrossRef]
- Biedron, T.; Szymanski, R.; Kubisa, P.; Penczek, S. Makromol. Chem, Macromol. Symp. 1990, 32, 155. [CrossRef]
- Lagarde, F.; Reibel, L; Franta, E. Makromol.Chem. 1992, 193, 1087. [CrossRef]
- Weiss, A. Angew. Chem. Int, Ed. 1981, 20, 850. [CrossRef]
- Wojtania, M.; Kubisa, P; Penczek, S. Makromol. Chem. Symp. 1986, 6, 201. [CrossRef]
- Kubisa, P. Makromol. Chem. Symp. 1988, 13/14, 203.
- Xu, Y.; Dong, S.; Fan, C.; Wang, W.; Den, K; Zhow, M. Z. Gaoefenzi Tongxun. 1981, 5, 368.
- Belbachir, M; Bensaoula, A. US Patent 6 274 527 B1, 2001.
- Meghabar, R.; Megherbi, A; Belbachir, M. Polymer. (in press).
- Ferrahi, M.I; Belbachir, M. Int. J. Mol. Sci. 2003, 4, 312. [CrossRef]
- Montmorillonite. Montmorillon (Vienne,France), Damour. An. Ph. Ch. 1847, 21, 376. [Google Scholar]
- Bentonite, Upton, Wyoming (USA), Analytical Data Reference, Clay Min, Report N° 7. Amer. Petro. Int. Project 49. 1950.
- Yun Kwon, O.; Won Park, K; Young Jeong, S. Bull. Korean Chem. Soc. 2001, 22(7), 679.
- Farmer, V. C. Infrared Spectra of Minerals; Farmer, V. C., Ed.; Mineralogical Society: London, 1974; p. 331. [Google Scholar]
- Moeke, H. H. W. Infrared Spectra of Minerals; Farmer, V. C., Ed.; Mineralogical Society: London, 1974; p. 365. [Google Scholar]
- Madejovà, J.; Bednànikovà, E.; Komadel, P; Cicel, B. in Proc.11 th Conf. Chem. Miner. Petrol.Ceske Budéjovica 1990; Konta, J., Ed.; Charles University: Prague, 1993; p. 267. [Google Scholar]
- Komadel, P. Clays Minerals 2003, 38, 127.
- Benharrats, N.; Belbachir, M.; Legran, A. P; D’espinose de le Caillerie, J. B. Clays Minerals 2003, 38, 49. [CrossRef]
- Samajovà, E.; Kraus, I; Lajcàkovà, A. Geol. Carpath. Ser. Clays. 1992, 42, 21.
- Tkàc, I.; Komadel, P; Müle, D. Clay Miner. 1994, 29, 11.
- Njopwouo, D.; Roques, G; Wandji, R. Clay Miner. 1987, 22, 45. [CrossRef]
- Kadokawa, J.; Iwasaki, Y; Tagaya, H. Green Chemistry. 2002, 4, 14. [CrossRef]
- Crivello, J. V; Fan, M. J. Polym. Sci. Part A 1992, 30, 1. [CrossRef]
- Oguni, N.; Maeda, S; Tani, H. Macromolecules 1973, 6, 459. [CrossRef]
- Ishimori, M.; Tsukigawa, T; Tsuruta, T. Makromol.Chem. 1976, 177, 1221. [CrossRef]
© 2003 by MDPI (http://www.mdpi.org). Reproduction for noncommercial purposes permitted.
Share and Cite
Yahiaoui, A.; Belbachir, M.; Hachemaoui, A. Cationic Polymerization of 1,2-Epoxypropane by an Acid Exchanged Montmorillonite Clay in the Presence of Ethylene Glycol. Int. J. Mol. Sci. 2003, 4, 572-585. https://doi.org/10.3390/i4110572
Yahiaoui A, Belbachir M, Hachemaoui A. Cationic Polymerization of 1,2-Epoxypropane by an Acid Exchanged Montmorillonite Clay in the Presence of Ethylene Glycol. International Journal of Molecular Sciences. 2003; 4(11):572-585. https://doi.org/10.3390/i4110572
Chicago/Turabian StyleYahiaoui, Ahmed, Mohammed Belbachir, and Aïcha Hachemaoui. 2003. "Cationic Polymerization of 1,2-Epoxypropane by an Acid Exchanged Montmorillonite Clay in the Presence of Ethylene Glycol" International Journal of Molecular Sciences 4, no. 11: 572-585. https://doi.org/10.3390/i4110572




