Electrical Conductivity along Phase Diagram of the Critical Mixture Isobutyric Acid – Water with Added (K+, Cl-) Ions
Abstract
:Introduction
The Coexistence Curves and the Transition Temperature
The transition temperature Tt
Experimental Details
Electrical conductivity measurement
Data Collection and Analysis
Electrical conductivity along the coexistence curve
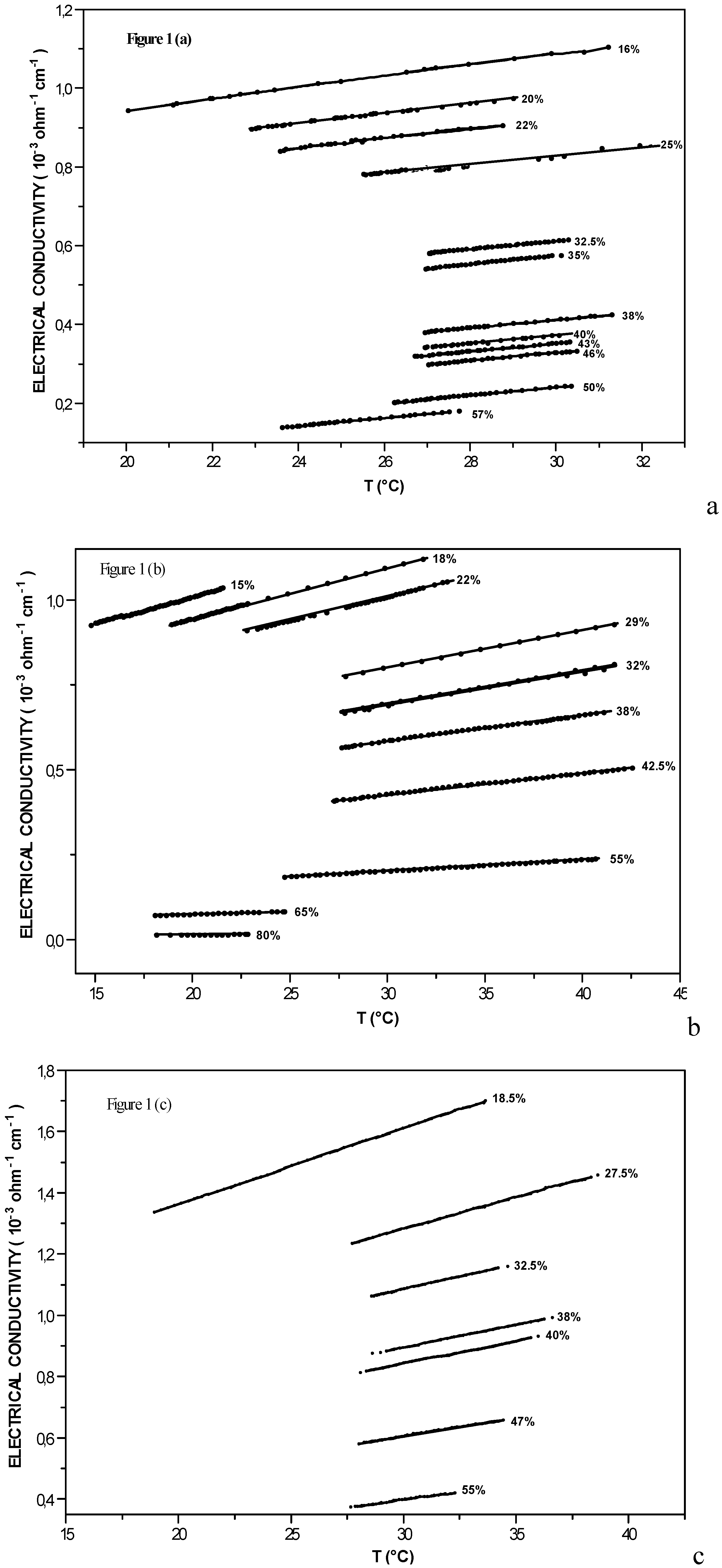
| X% in acid | (I-W) | (I-W) + 5 10-4 M [KCl] | (I-W) + 5 10-3 M [KCl] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tt (°C) | σx | Ax10+3 | Tt (°C) | σx | Ax10+3 | Tt (°C) | σx | Ax10+3 | |
| 15 | 15.063 | -3.58 | 15.68 | ||||||
| 16 | 19.992 | -3.23 | 14.27 | ||||||
| 18 | 18.820 | -3.48 | 15.09 | ||||||
| 18.5 | 17.125 | -5.91 | 24.82 | ||||||
| 20 | 22.842 | -2.78 | 12.42 | ||||||
| 22 | 23.527 | -2.85 | 11.96 | 22.710 | -3.29 | 14.18 | |||
| 25 | 25.547 | -2.58 | 11.34 | ||||||
| 27.5 | - | 26.649 | -4.93 | 20.51 | |||||
| 29 | 26.353 | -2.56 | 11.09 | ||||||
| 30 | 26.930 | -2.26 | 10.9 | ||||||
| 32 | 27.014 | -2.31 | 9.9 | ||||||
| 32.5 | 27.057 | -2.46 | 10.7 | 28.021 | -3.88 | 16.39 | |||
| 35 | 26.962 | -2.79 | 10.34 | ||||||
| 38 | 26.945 | -2.67 | 10.17 | 27.356 | -1.69 | 7.52 | 28.516 | -3.58 | 14.78 |
| 40 | 26.904 | -2.66 | 10.12 | 28.091 | -3.61 | 14.1 | |||
| 42 | 27.018 | -1.46 | 6.24 | ||||||
| 43 | 26.841 | -2.72 | 10.06 | ||||||
| 46 | 26.750 | -2.71 | 10.03 | ||||||
| 47 | 28.012 | -3.14 | 12.37 | ||||||
| 50 | 26.217 | -2.88 | 10.02 | ||||||
| 55 | 24.803 | -0.871 | 3.52 | 27.645 | -2.72 | 10.31 | |||
| 57 | 23.520 | -2.83 | 10.01 | ||||||
| 62.5 | 21.984 | -1.01 | |||||||
| 65 | 14.390 | -0.46 | 1.84 | ||||||


- (i)
- ( ∂σ/∂T )(I-W) + 5 10-4 M [KCl] = 7.52 10-3 Ω-1cm-1K-1
- (ii)
- ( ∂σ/∂T )(I-W) + 5 10-3 M [KCl] = 14.78 10-3 Ω-1cm-1K-1
Conclusion
References
- Toumi, A.; Bouanz, M. Critical behavior of the binary-fluid isobutyric acid-water with added ions (K+, Cl-). Eur. Phys. J. 2000, E 2, 211–216. [Google Scholar]
- Toumi, A.; Bouanz, M.; Gharbi, A. Coexistence curves of the binary mixture isobutyric acid-water with added ions (K+, Cl-). Chem. Phys. Lett. 2002, 362, 567–573. [Google Scholar] [CrossRef]
- Leguillou, J. C.; Zinn-Justin, J. Critical exponents from field theory. Phys. Rev. 1981, B21, 3976–3998. [Google Scholar]
- Green, M. S.; Ley-Koo, M. Phys. Rev. 1981, A23, 2650.
- Wegner, F. J. Corrections to scaling laws. Phys. Rev. 1972, B5, 4529–4536. [Google Scholar] [CrossRef]
- Beysens, D.; Bourgou, A.; Calmettes, P. Experimental determination of universal amplitude combinations for binary fluids. I. Static. Phys. Rev. 1982, A29, 3589–3609. [Google Scholar] [CrossRef]
- Hohenberg, P. C.; Bartmatz, M. Gravity effects near the gas-liquid critical point. Phys. Rev. 1972, A46, 289–313. [Google Scholar] [CrossRef]
- Bouanz, M.; Beysens, D. Effect of ion impurities on a binary mixture of isobutyric acid and water. Chem. Phys. Lett. 1994, 231, 105–110. [Google Scholar] [CrossRef]
- Bouanz, M. Critical behavior of the self-diffusion coefficient in a binary fluid. Phys. Rev. 1992, A46, 4888–4893. [Google Scholar] [CrossRef]
- Bouanz, M. The solvation phenomenon in a binary fluid. Quimica Analitica 1996, 15. SS (30-33). [Google Scholar]
© 2003 by MDPI (http://www.mdpi.org). Reproduction for noncommercial purposes permitted.
Share and Cite
Cherif, E.; Bouanz, M. Electrical Conductivity along Phase Diagram of the Critical Mixture Isobutyric Acid – Water with Added (K+, Cl-) Ions. Int. J. Mol. Sci. 2003, 4, 326-334. https://doi.org/10.3390/i4060326
Cherif E, Bouanz M. Electrical Conductivity along Phase Diagram of the Critical Mixture Isobutyric Acid – Water with Added (K+, Cl-) Ions. International Journal of Molecular Sciences. 2003; 4(6):326-334. https://doi.org/10.3390/i4060326
Chicago/Turabian StyleCherif, Emna, and Moncef Bouanz. 2003. "Electrical Conductivity along Phase Diagram of the Critical Mixture Isobutyric Acid – Water with Added (K+, Cl-) Ions" International Journal of Molecular Sciences 4, no. 6: 326-334. https://doi.org/10.3390/i4060326




