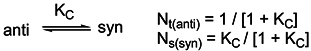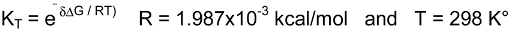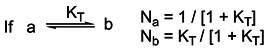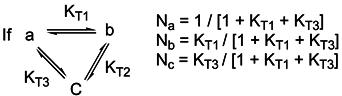Basicity
It is well known that it is impossible to measure the pKa values of the individual tautomers in the tautomeric equilibrium and it is usually difficult to prepare all the fixed model compounds of the individual tautomers in which the mobile H-atom replaced with a generally methyl group to eliminate the migration of H-atom. In aqueous solution the acidity of a given base B is the standard free energy change calculated by means of Eq. 7 for the reaction 6.
AM1 and PM5 calculated heats of formation and absolute entropy values of H
3O+ and H
2O in aqueous solution are given in
Table 1,
Table 2,
Table 3 and
Table 4. These were converted to free energy by using AM1 and PM5 calculated absolute entropy values. In aqueous solution the pK
a values of the studied molecules were found by using the following equation
Where R= 1.987x10-3 kcal/mol °K and T= 298 °K. In this work pKa values of various 3-substituted pyridine derivatives were calculated in aqueous media by means of AM1 COSMO method in MOPAC2000 and PM5 COSMO method in MOPAC2002. All pKa values calculated by Eq. 8 are listed in
Table 3 and
Table 6. As can be seen from
Table 3 and
Table 6 the obtained pKa values from AM1 and PM5 calculations are generally in very good agreement with the experimental pKa values. The absolute error between experimental and calculated pKa values are within ± 0-1 kcal/mol range, exceptions of conjugate bases 15, 21-23, and 36, with AM1 calculations i.e., ± 0-2 kcal/mol, and conjugate bases 13, 15-16, 22, and 31 with PM5 calculations.
Both AM1 and PM5 overestimated the pK
a value of the 3-mercaptopyridine 15 approximately in 5 pKa units giving the figures 10.58 and 10.90, respectively. Experimentally 3-aminopyridine 22 in aqueous solution undergoes predominant protonation at the ring nitrogen atom [
11]. In both AM1 and PM5 calculations the protonation of 22 found to be predominantly takes place at exocyclic nitrogen atom giving cation 58b, which is not consistent with experimental data. However, the protonation of 23 occurs predominantly at the ring nitrogen atom in AM1 calculations and mostly at the exocyclic nitrogen atom in PM5 resulting in cation 59a. Due to these results, probably, the acidity constant of the 3-aminopyridine 22 was found to be 5 pK
a units less than the experimental value by both AM1 and PM5. While the PM5 could calculate the pK
a value for 3-cyanopyrine 36 which is very close to experimental value being approximately 1.35. However, the AM1 overestimated value of 4.21 was obtained by AM1. By AM1 method the pK
a value for the conjugate base 31 was found to be as 3.32, which is consistent with the experimental values 3.13 or 3.75, but PM5 predicted the molecule 31 as much more acidic compared to the experimental data. The acidity constants for the 3-nitropyridine 21 and 3-dimethylaminopyrine 23 predicted by means of AM1 COSMO method exceeds the acceptable limit ± 0-1 pK
a unit.
Table 1.
The AM1 calculated thermodynamic properties of pyridine derivatives in aqueous solution (ε=78.4).
Table 1.
The AM1 calculated thermodynamic properties of pyridine derivatives in aqueous solution (ε=78.4).
| Compound | ΔHf (kcal/mol) | ΔS (cal/mol K) | ΔGf a (kcal/mol) | Mol fractions of conformers or tautomersb | Weighted average ΔGf c (kcal/mol) |
|---|
| 1 | 23.81 | 67.41 | 3.72 | | |
| 2 | 15.82 | 75.27 | -6.61 | | |
| 3 | 9.99 | 84.08 | -15.07 | | |
| 4 | 5.94 | 90.20 | -20.94 | | |
| 6s | -35.14 | 78.17 | -58.43 | N6s = 0.08 | -59.74 |
| 6t | -35.12 | 83.00 | -59.85 | N6t = 0.92 | |
| 11 | -48.81 | 101.49 | -79.05 | | |
| 13a | -24.83 | 74.70 | -47.10 | N13a = 1.00 | -47.10 |
| 13b | -21.69 | 73.35 | -43.55 | N13b = 0.00 | |
| 14s | -17.00 | 81.44 | -41.27 | N14s = 0.49 | -41.28 |
| 14t | -16.82 | 82.11 | -41.29 | N14t = 0.51 | |
| 15a | 23.47 | 79.34 | -0.17 | N15a = 0.00 | -9.62 |
| 15b | 13.15 | 76.40 | -9.62 | N15b = 1.00 | |
| 16a | -138.95 | 90.49 | -165.92 | N16a = 0.99 | -165.89 |
| 16b | -135.16 | 94.22 | -163.24 | N16b = 0.01 | |
| 17 | -21.00 | 73.05 | -42.77 | | |
| 18 | 16.94 | 75.78 | -5.64 | | |
| 19 | 27.89 | 78.72 | 4.43 | | |
| 20 | 39.32 | 80.62 | 15.30 | | |
| 21 | 17.16 | 84.73 | -8.09 | | |
| 22a | 15.58 | 74.66 | -6.67 | N22a = 1.00 | -6.67 |
| 22b | 39.53 | 76.79 | 16.65 | N22b = 0.00 | |
| 23 | 28.09 | 90.57 | 1.10 | | |
| 24a | -25.52 | 88.95 | -52.03 | N24a = 0.10 | -52.72 |
| 24b | -24.92 | 91.95 | -51.60 | N24b = 0.05 | |
| 24c | -25.04 | 93.73 | -52.97 | N24c = 0.52 | |
| 24d | -25.35 | 91.78 | -52.70 | N24d = 0.33 | |
| 25a | -16.06 | 100.30 | -45.95 | N25a = 0.32 | -45.82 |
| 25b | -15.62 | 100.88 | -45.68 | N25b = 0.20 | |
| 25c | -15.67 | 100.63 | -45.66 | N25c = 0.20 | |
| 25d | -16.00 | 100.25 | -45.87 | N25d = 0.28 | |
| 28s | -14.56 | 80.42 | -38.53 | N28s = 0.51 | -38.52 |
| 28t | -14.58 | 80.26 | -38.50 | N28t = 0.49 | |
| 29s | -20.40 | 91.34 | -47.62 | N29s = 0.82 | -47.46 |
| 29t | -20.34 | 88.53 | -46.73 | N29t = 0.18 | |
| 31a | -77.03 | 86.63 | -102.84 | N31a = 0.84 | -102.62 |
| 31bs | -75.82 | 85.56 | -101.32 | N31b = 0.05 | |
| 31bt | -74.81 | 85.85 | -100.39 | N31c = 0.11 | |
| 31cs | -75.84 | 86.97 | -101.76 | | |
| 31ct | -74.62 | 86.45 | -100.38 | | |
| 32 | -173.27 | 86.39 | -199.01 | | |
| 33ss | -66.86 | 98.59 | -96.24 | N33ss = 0.98 | -96.21 |
| 33st | -64.15 | 93.94 | -92.14 | N33st = 0.00 | |
| 33ts | -66.89 | 90.99 | -94.01 | N33ts = 0.02 | |
| 33tt | -64.07 | 94.50 | -92.23 | N33tt = 0.00 | |
| 34ss | -72.52 | 103.66 | -103.41 | N34ss = 0.68 | -103.22 |
| 34st | -69.89 | 105.79 | -101.42 | N34st = 0.02 | |
| 34ts | --72.56 | 101.89 | -102.92 | N34ts = 0.30 | |
| 34tt | -69.84 | 102.97 | -100.53 | N34tt = 0.00 | |
| 35s | -28.56 | 85.54 | -54.06 | N35s = 0.54 | -54.01 |
| 35t | -28.50 | 85.45 | -53.96 | N35t = 0.46 | |
| 36 | 52.35 | 77.61 | 29.22 | | |
| H2O | -68.89 | 45.11 | -82.31 | | |
Table 2.
The AM1 calculated thermodynamic properties of protonated pyridine derivatives in aqueous solution (ε=78.4)
Table 2.
The AM1 calculated thermodynamic properties of protonated pyridine derivatives in aqueous solution (ε=78.4)
| Compound | ΔHf (kcal/mol) | ΔS (cal/mol K) | ΔGfa (kcal/mol | Mol fractions of conformers or tautomersb | Weighted average ΔGf c (kcal/mol) |
|---|
| 37 | 118.38 | 67.77 | 98.18 | | |
| 38 | 111.04 | 80.48 | 87.06 | | |
| 39 | 105.20 | 85.55 | 79.71 | | |
| 40 | 101.20 | 92.26 | 73.71 | | |
| 42s | 60.17 | 78.45 | 36.81 | N42s = 0.04 | 35.07 |
| 42t | 60.09 | 84.19 | 35.00 | N42t = 0.96 | |
| 47 | 44.81 | 100.39 | 14.89 | | |
| 49 | 71.25 | 75.35 | 48.80 | | |
| 50s | 79.85 | 83.70 | 54.91 | N50s = 0.25 | 54.43 |
| 50t | 80.03 | 86.45 | 54.27 | N50t = 0.75 | |
| 51 | 119.55 | 80.48 | 95.57 | | |
| 52 | -40.25 | 96.02 | -68.86 | | |
| 53 | 75.96 | 73.50 | 54.06 | | |
| 54 | 113.65 | 76.24 | 90.93 | | |
| 55 | 124.49 | 79.15 | 100.90 | | |
| 56 | 135.60 | 81.00 | 111.46 | | |
| 57a | 115.06 | 84.23 | 89.96 | N57a = 1.00 | 89.96 |
| 57b | 141.24 | 84.66 | 116.01 | N57b = 0.00 | |
| 57c | 141.23 | 83.44 | 116.36 | N57c = 0.00 | |
| 58a | 112.39 | 75.87 | 89.79 | N58a = 0.00 | 80.59 |
| 58b | 103.51 | 76.92 | 80.59 | N58b = 1.00 | |
| 59a | 124.79 | 92.13 | 97.34 | N59a = 1.00 | 97.34 |
| 59b | 128.49 | 90.59 | 101.49 | N59b = 0.00 | |
| 60a | 71.08 | 88.65 | 44.68 | N60a = 0.01 | 42.39 |
| 60b | 71.27 | 94.16 | 43.21 | N60b = 0.13 | |
| 60c | 70.99 | 92.11 | 43.54 | N60c = 0.07 | |
| 60d | 70.76 | 96.12 | 42.12 | N60d = 0.79 | |
| 61a | 80.63 | 96.86 | 51.77 | N61a = 0.08 | 50.84 |
| 61b | 80.77 | 101.54 | 50.51 | N61b = 0.70 | |
| 61c | 80.58 | 97.09 | 51.65 | N61c = 0.10 | |
| 61d | 80.46 | 96.54 | 51.69 | N61d = 0.11 | |
| 64s | 81.23 | 80.93 | 57.11 | N64s = 0.41 | 56.99 |
| 64t | 80.96 | 80.74 | 56.90 | N64t = 0.59 | |
| 65s | 75.63 | 89.30 | 49.02 | N65s = 0.56 | 49.08 |
| 65t | 75.60 | 88.73 | 49.16 | N65t = 0.44 | |
| 67ss | 20.19 | 86.90 | -5.71 | N67ss = 0.50 | -5.47 |
| 67st | 21.06 | 86.73 | -4.79 | N67st = 0.11 | |
| 67ts | 20.05 | 85.75 | -5.50 | N67ts = 0.35 | |
| 67tt | 21.00 | 84.54 | -4.19 | N67tt = 0.04 | |
| 68ss | 29.32 | 95.36 | 0.90 | N68ss = 0.82 | 1.06 |
| 68st | 32.03 | 94.92 | 3.74 | N68st = 0.00 | |
| 68ts | 29.13 | 91.75 | 1.79 | N68ts = 0.18 | |
| 68tt | 32.03 | 95.30 | 3.63 | N68tt = 0.01 | |
| 69ss | 23.79 | 96.89 | -5.08 | N69ss = 0.03 | -6.80 |
| 69st | 26.93 | 97.75 | -2.20 | N69st = 0.00 | |
| 69ts | 23.58 | 102.34 | -6.92 | N69ts = 0.96 | |
| 69tt | 26.25 | 103.35 | -4.55 | N69tt = 0.01 | |
| 70s | 67.66 | 85.71 | 42.12 | N70s = 0.49 | 42.10 |
| 70t | 67.38 | 85.73 | 42.09 | N70t = 0.51 | |
| 71 | 148.47 | 78.16 | 125.18 | | |
| H3O+ | 33.13 | 46.13 | 19.38 | | |
Table 3.
The AM1 calculated pKa values for pyridines in aqueous solution (ε=78.4).
Table 3.
The AM1 calculated pKa values for pyridines in aqueous solution (ε=78.4).
| Conjugate base (B) | ΔGf Kcal/mol | Conjugate acid (BH+) | ΔGf Kcal/mol | δΔGfa Kcal/mol | pKa(BH+)b | Exp. pKa(BH+)c | Absolute error between pKa(calc.) and pKa(exp.) | Referencesc |
|---|
| 1 | 3.72 | 37 | 98.18 | 7.23 | 5.30 | 5.27, 5.28 | 0.03, 0.02 | 23, 24, 25 |
| 2 | -6.61 | 38 | 87.06 | 8.02 | 5.88 | 5.67, 5.70, 5.75 | 0.21, 0.18, 0.13 | 26, 27, 28, 29 |
| 3 | -15.07 | 39 | 79.71 | 6.91 | 5.07 | 5.70 | 0.63 | 30 |
| 4 | -20.94 | 40 | 73.71 | 7.04 | 5.16 | 5.72 | -0.56 | 30 |
| 6 | -59.74 | 42 | 35.07 | 6.88 | 5.05 | 4.90, 4.95 | 0.15, 0.10 | 31, 32 |
| 11 | -79.05 | 47 | 14.89 | 7.75 | 5.68 | 5.47 | 0.21 | 31 |
| 13 | -47.10 | 49 | 48.80 | 5.79 | 4.25 | 4.80, 4.86 | -0.55, -0.61 | 27, 38, 39, 40 |
| 14 | -41.28 | 50 | 54.43 | 5.98 | 4.39 | 4.78, 4.88, 4.90 | -0.39, -0.49, -0.51 | 26, 40, 38, 27 |
| 15 | -9.62 | 51 | 95.57 | -3.5 | -2.57 | 2.28 | -4.85 | 19 |
| 16 | -165.89 | 52 | -68.86 | 4.66 | 3.42 | 3.22 | 0.20 | 41 |
| 17 | -42.77 | 53 | 54.06 | 4.86 | 3.56 | 2.97, 3.10 | 0.59, 0.46 | 30, 42 |
| 18 | -5.64 | 54 | 90.93 | 5.12 | 3.75 | 2.81, 2.84, 2.98 | 0.94, 0.91, 0.77 | 26, 42, 27 |
| 19 | 4.43 | 55 | 100.90 | 5.22 | 3.83 | 2.80, 2.84, 2.85 | 1.03, 0.99, 0.98 | 27, 42, 26 |
| 20 | 15.30 | 56 | 111.46 | 5.53 | 4.05 | 3.25 | 0.85 | 30 |
| 21 | -8.09 | 57 | 89.96 | 3.64 | 2.67 | 0.81, 1.18 | 1.86, 1.49 | 43, 26 |
| 22 | -6.67 | 58 | 80.59 | 14.43 | 10.58 | 5.80, 5.98, 6.04 | 4.78, 4.60, 4.54 | 30, 44, 26, 27 |
| 23 | 1.10 | 59 | 97.34 | 5.38 | 3.95 | 6.45 | -2.50 | 45 |
| 24 | -52.72 | 60 | 42.39 | 6.58 | 4.83 | 4.46 | 0.37 | 46 |
| 25 | -45.82 | 61 | 50.84 | 5.03 | 3.69 | 3.52 | 0.17 | 46 |
| 28 | -38.52 | 64 | 56.99 | 6.18 | 4.53 | 3.70, 3.75 | 0.83, 0.78 | 47, 48 |
| 29 | -47.46 | 65 | 49.08 | 5.15 | 3.78 | 3.18, 3.26 | 0.60, 0.52 | 30, 49 |
| 31 | -102.62 | 67 | -5.47 | 4.53 | 3.32 | 3.13, 3.75 | 0.19, -0.43 | 43, 50 |
| 32 | -199.01 | 31 | -102.62 | 5.30 | 3.89 | 4.77 | -0.88 | 51 |
| 33 | -96.21 | 68 | 1.06 | 4.42 | 3.24 | 3.09 | 0.15 | 26 |
| 34 | -103.22 | 69 | -6.80 | 5.27 | 3.86 | 3.35 | 0.51 | 42 |
| 35 | -54.01 | 70 | 42.10 | 5.58 | 4.09 | 3.40 | 0.69 | 51 |
| 36 | 29.22 | 71 | 125.18 | 5.74 | 4.21 | 1.30, 1.36, 1.45 | 2.91, 2.85, 2.76 | 27, 26, 52, 51 |
| H2O | -82.31 | H3O+ | 19.38 | | | | | |
Table 4.
The PM5 calculated thermodynamic properties of pyridine derivatives in aqueous solution (ε=78.4).
Table 4.
The PM5 calculated thermodynamic properties of pyridine derivatives in aqueous solution (ε=78.4).
| Compound | ΔHf (Kcal/mol) | ΔS (cal/mol K) | ΔGf a (Kcal/mol) | Mol fractions of conformers or tautomersb | Weighted average ΔGf c (Kcal/mol) |
|---|
| 1 | 23.20 | 68.11 | 2.90 | | |
| 2 | 14.89 | 76.07 | -7.78 | | |
| 3 | 9.86 | 86.55 | -15.93 | | |
| 4 | 4.38 | 93.33 | -23.48 | | |
| 5 | -0.41 | 97.49 | -29.46 | | |
| 6s | -25.82 | 86.73 | -51.67 | N6s = 0.09 | -52.93 |
| 6t | -27.77 | 84.82 | -53.05 | N6t = 0.91 | |
| 7 | 16.47 | 112.82 | -17.15 | | |
| 8s | -1.02 | 115.97 | -35.58 | N8a = 0.57 | -35.51 |
| 8t | -0.97 | 115.61 | -35.42 | N8b = 0.43 | |
| 9s | -80.34 | 109.42 | -112.95 | N9a = 0.44 | -113.03 |
| 9t | -79.82 | 111.68 | -113.10 | N9b = 0.56 | |
| 10s | -40.59 | 121.95 | -76.93 | N10a = 0.00 | -82.08 |
| 10t | -45.00 | 124.42 | -82.08 | N10b = 1.00 | |
| 11 | -39.97 | 101.01 | -70.07 | | |
| 12 | 57.30 | 110.02 | 24.51 | | |
| 13a | -23.18 | 75.95 | -45.81 | N13a = 0.00 | -50.34 |
| 13b | -28.23 | 74.19 | -50.34 | N13b = 1.00 | |
| 14s | -14.94 | 83.20 | -39.74 | N14s = 0.28 | -40.14 |
| 14t | -15.09 | 84.59 | -40.3 | N14t = 0.72 | |
| 15a | 26.92 | 81.95 | 2.50 | N15a = 0.00 | -5.68 |
| 15b | 17.32 | 77.17 | -5.68 | N15b = 1.00 | |
| 16a | -126.62 | 92.81 | -154.28 | N16a = 1.00 | -154.28 |
| 16b | -112.87 | 93.42 | -140.71 | N16b = 0.00 | |
| 17 | -21.66 | 73.79 | -43.65 | | |
| 18 | 16.33 | 76.76 | -6.54 | | |
| 19 | 27.51 | 79.75 | 3.74 | | |
| 20 | 40.88 | 82.31 | 16.35 | | |
| 21 | 14.89 | 84.82 | -10.39 | | |
| 22a | 16.69 | 76.03 | -5.96 | N22a = 1.00 | -5.96 |
| 22b | 37.36 | 78.10 | 14.09 | N22b = 0.00 | |
| 23 | 27.40 | 91.44 | 0.15 | | |
| 24a | -32.65 | 93.58 | -60.54 | N24a = 0.41 | -60.32 |
| 24b | -31.30 | 94.64 | -59.50 | N24b = 0.08 | |
| 24c | -31.37 | 96.57 | -60.15 | N24c = 0.21 | |
| 24d | -32.49 | 93.49 | -60.35 | N24d = 0.30 | |
| 25a | -26.49 | 102.59 | -57.06 | N25a = 0.18 | -57.31 |
| 25b | -25.71 | 107.07 | -57.62 | N25b = 0.47 | |
| 25c | -25.70 | 104.98 | -56.98 | N25c = 0.17 | |
| 25d | -26.36 | 103.04 | -57.06 | N25d = 0.18 | |
| 26a | -2.11 | 113.23 | -35.85 | N26a = 0.60 | -35.62 |
| 26b | -0.69 | 111.98 | -34.06 | N26b = 0.03 | |
| 26c | -0.64 | 112.57 | -34.19 | N26c = 0.04 | |
| 26d | -1.97 | 112.55 | -35.51 | N26d = 0.33 | |
| 27a | 4.50 | 125.10 | -32.78 | N27a = 0.54 | -32.56 |
| 27b | 4.95 | 121.27 | -31.19 | N27b = 0.04 | |
| 27c | 5.02 | 121.98 | -31.33 | N27c = 0.05 | |
| 27d | 4.68 | 124.97 | -32.56 | N27d = 0.37 | |
| 28s | -12.90 | 80.90 | -37.01 | N28s = 0.50 | -37.01 |
| 28t | -12.81 | 81.17 | -37.00 | N28t = 0.50 | |
| 29s | -23.98 | 88.93 | -50.48 | N29s = 0.59 | -50.39 |
| 29t | -23.88 | 88.52 | -50.26 | N29t = 0.41 | |
| 30s | 6.57 | 105.44 | -24.85 | N30s = 0.47 | -24.89 |
| 30t | 6.46 | 105.33 | -24.93 | N30t = 0.53 | |
| 31a | -84.13 | 85.45 | -109.59 | N31a = 1.00 | -109.59 |
| 31bs | -72.64 | 86.22 | -98.33 | N31b = 0.00 | |
| 31bt | -71.99 | 86.04 | -97.62 | N31c = 0.00 | |
| 31cs | -72.63 | 85.32 | -98.06 | | |
| 31ct | -70.93 | 81.13 | -95.11 | | |
| 32 | -186.31 | 79.52 | -210.13 | | |
| 33ss | -65.43 | 94.48 | -93.59 | N33ss = 0.48 | -93.52 |
| 33st | -63.30 | 95.82 | -91.85 | N33st = 0.03 | |
| 33ts | -65.43 | 94.52 | -93.60 | N33ts = 0.48 | |
| 33tt | -63.16 | 95.20 | -91.53 | N33tt = 0.01 | |
| 34ss | -71.88 | 103.55 | -102.74 | N34ss = 0.65 | -102.49 |
| 34st | -70.05 | 103.73 | -100.96 | N34st = 0.03 | |
| 34ts | -71.86 | 102.01 | -102.26 | N34ts = 0.29 | |
| 34tt | -69.86 | 104.01 | -100.85 | N34tt = 0.03 | |
| 35s | -34.15 | 86.57 | -59.95 | N35s = 0.61 | -59.85 |
| 35t | -34.02 | 86.13 | -59.69 | N35t = 0.39 | |
| 36 | 52.32 | 78.49 | 28.93 | | |
| H2O | -59.44 | 44.99 | -72.85 | | |
Table 5.
The PM5 calculated thermodynamic properties of protonated pyridine derivatives in aqueous solution (ε=78.4).
Table 5.
The PM5 calculated thermodynamic properties of protonated pyridine derivatives in aqueous solution (ε=78.4).
| Compound | ΔHf (Kcal/mol) | ΔS (cal/mol K) | ΔGf a (Kcal/mol | Mol fractions of conformers or tautomersb | Weighted average ΔGf c (Kcal/mol) |
|---|
| 37 | 121.80 | 68.59 | 101.36 | | |
| 38 | 113.73 | 79.14 | 90.15 | | |
| 39 | 109.00 | 86.50 | 83.22 | | |
| 40 | 103.32 | 93.27 | 75.53 | | |
| 41 | 98.58 | 96.84 | 69.72 | | |
| 42s | 72.67 | 86.97 | 46.75 | N42s = 0.53 | 46.78 |
| 42t | 72.66 | 86.73 | 46.81 | N42t = 0.47 | |
| 43a | 115.66 | 113.65 | 81.79 | N43a = 0.00 | 1.00 |
| 43b | 109.85 | 112.55 | 76.31 | N43b = 1.00 | |
| 44s | 99.68 | 116.00 | 65.11 | N44s = 0.26 | 64.64 |
| 44t | 99.60 | 117.86 | 64.48 | N44t = 0.74 | |
| 45s | 20.63 | 113.10 | -13.07 | N45s = 0.71 | -12.92 |
| 45t | 20.69 | 111.56 | -12.55 | N45t = 0.29 | |
| 46s | 61.58 | 122.15 | 25.18 | N46s = 0.00 | |
| 46t | 56.29 | 122.48 | 19.79 | N46t = 1.00 | |
| 47 | 59.29 | 101.27 | 29.11 | | |
| 48 | 156.51 | 109.33 | 123.93 | | |
| 49 | 77.90 | 75.96 | 55.26 | | |
| 50s | 86.40 | 87.29 | 60.39 | N50s = 0.98 | 60.44 |
| 50t | 86.71 | 80.44 | 62.74 | N50t = 0.02 | |
| 51 | 127.96 | 81.08 | 103.80 | | |
| 52 | -6.43 | 94.86 | -34.70 | | |
| 53 | 81.79 | 74.22 | 59.67 | | |
| 54 | 118.24 | 77.23 | 95.23 | | |
| 55 | 129.46 | 80.21 | 105.56 | | |
| 56 | 141.60 | 82.75 | 116.95 | | |
| 57a | 122.34 | 85.33 | 96.91 | N57a = 1.00 | 96.91 |
| 57b | 152.00 | 85.62 | 126.49 | N57b = 0.00 | |
| 57c | 151.94 | 86.06 | 126.29 | N57c = 0.00 | |
| 58a | 115.72 | 76.66 | 92.88 | N58a = 0.00 | 85.14 |
| 58b | 108.61 | 78.75 | 85.14 | N58b = 1.00 | |
| 59a | 127.09 | 92.35 | 99.57 | N59a = 0.71 | 99.72 |
| 59b | 127.52 | 92.01 | 100.10 | N59b = 0.29 | |
| 60a | 69.61 | 96.97 | 40.71 | N60a = 0.58 | 40.79 |
| 60b | 71.11 | 94.92 | 42.82 | N60b = 0.00 | |
| 60c | 70.92 | 94.36 | 42.80 | N60c = 0.00 | |
| 60d | 69.46 | 95.86 | 40.89 | N60d = 0.42 | |
| 61a | 76.06 | 103.79 | 45.13 | N61a = 0.47 | 45.33 |
| 61b | 76.84 | 99.34 | 47.24 | N61b = 0.01 | |
| 61c | 76.75 | 104.25 | 45.68 | N61c = 0.19 | |
| 61d | 75.93 | 102.62 | 45.35 | N61d = 0.33 | |
| 62a | 100.67 | 115.08 | 66.37 | N62a = 0.71 | 66.59 |
| 62b | 102.48 | 112.86 | 68.85 | N62b = 0.01 | |
| 62c | 101.96 | 112.37 | 68.47 | N62c = 0.02 | |
| 62d | 100.51 | 112.60 | 66.97 | N62d = 0.26 | |
| 63a | 107.58 | 122.27 | 71.14 | N63a = 0.15 | 70.46 |
| 63b | 108.32 | 122.08 | 71.94 | N63b = 0.04 | |
| 63c | 107.81 | 121.03 | 71.74 | N63c = 0.05 | |
| 63d | 107.41 | 124.95 | 70.17 | N63d = 0.76 | |
| 64s | 90.19 | 81.96 | 65.76 | N64s = 0.46 | 65.71 |
| 64t | 90.14 | 82.15 | 65.66 | N64t = 0.54 | |
| 65s | 78.97 | 89.14 | 52.41 | N65s = 0.38 | 52.23 |
| 65t | 78.90 | 89.85 | 52.12 | N65t = 0.62 | |
| 66s | 110.11 | 109.01 | 77.63 | N66s = 0.64 | 77.75 |
| 66t | 109.97 | 107.36 | 77.97 | N66t = 0.36 | |
| 67ss | 31.76 | 86.71 | 5.92 | N67ss = 0.35 | 5.95 |
| 67st | 32.52 | 86.50 | 6.74 | N67st = 0.09 | |
| 67ts | 31.65 | 87.04 | 5.71 | N67ts = 0.50 | |
| 67tt | 32.66 | 86.12 | 7.00 | N67tt = 0.06 | |
| 68ss | 39.09 | 95.33 | 10.68 | N68ss = 0.40 | 10.77 |
| 68st | 41.15 | 97.48 | 12.10 | N68st = 0.04 | |
| 68ts | 39.01 | 95.71 | 10.49 | N68ts = 0.55 | |
| 68tt | 41.17 | 96.63 | 12.37 | N68tt = 0.02 | |
| 69ss | 31.32 | 105.80 | -0.21 | N69ss = 0.97 | -0.15 |
| 69st | 34.51 | 98.56 | 5.14 | N69st = 0.00 | |
| 69ts | 33.75 | 106.83 | 1.91 | N69ts = 0.03 | |
| 69tt | 34.56 | 103.65 | 3.67 | N69tt = 0.00 | |
| 70s | 68.87 | 87.07 | 42.92 | N70s = 0.53 | 42.95 |
| 70t | 68.85 | 86.82 | 42.98 | N70t = 0.47 | |
| 71 | 155.79 | 78.92 | 132.27 | | 133.05 |
| H3O+ | 46.81 | 45.91 | 33.13 | | |
Table 6.
The PM5 calculated pKa values for pyridines in aqueous solution (ε=78.4).
Table 6.
The PM5 calculated pKa values for pyridines in aqueous solution (ε=78.4).
| Conjugate base (B) | ΔGf kcal/mol | Conjugate acid (BH+) | ΔGf kcal/mol | δΔGf a kcal/mol | pKa(BH+)b | Exp. pKa(BH+)c | Absolute error between pKa(calc.) and pKa(exp.) | Referencesc |
|---|
| 1 | 2.90 | 37 | 101.36 | 7.52 | 5.51 | 5.27, 5.28 | 0.24, 0.23 | 23, 24, 25 |
| 2 | -7.78 | 38 | 90.15 | 8.05 | 5.90 | 5.67, 5.70, 5.75 | 0.23, 0.20, 0.15 | 26, 27, 28, 29 |
| 3 | -15.93 | 39 | 83.22 | 6.83 | 5.01 | 5.70 | -0.69 | 30 |
| 4 | -23.48 | 40 | 75.53 | 6.97 | 5.11 | 5.72 | -0.61 | 30 |
| 5 | -29.46 | 41 | 69.72 | 6.80 | 4.99 | 5.82 | -0.83 | 30 |
| 6 | -52.93 | 42 | 46.78 | 6.27 | 4.59 | 4.90, 4.95 | -0.31, -0.36 | 31, 32 |
| 7 | -17.15 | 43 | 76.31 | 12.52 | 9.18 | 8.55 | 0.63 | 33 |
| 8 | -35.51 | 44 | 64.64 | 5.83 | 4.28 | 4.87 | -0.59 | 34 |
| 9 | -113.03 | 45 | -12.92 | 5.87 | 4.30 | 4.67 | -0.37 | 35 |
| 10 | -82.08 | 46 | 19.79 | 4.11 | 3.01 | 3.67 | -0.66 | 36 |
| 11 | -70.07 | 47 | 29.11 | 6.80 | 4.98 | 5.47 | -0.49 | 31 |
| 12 | 24.51 | 48 | 123.93 | 6.56 | 4.81 | 4.75 | 0.06 | 37 |
| 13 | -50.34 | 49 | 55.26 | 0.38 | 0.28 | 4.80, 4.86 | -4.52, -4.58 | 27, 38, 39, 40 |
| 14 | -40.14 | 50 | 60.44 | 5.40 | 3.96 | 4.78, 4.88, 4.90 | -0.82, -0.92, -0.94 | 26, 40, 38, 27 |
| 15 | -5.68 | 51 | 103.80 | -3.50 | -2.56 | 2.28 | -4.84 | 19 |
| 16 | -154.28 | 52 | -34.70 | -13.60 | -9.97 | 3.22 | -13.19 | 41 |
| 17 | -43.65 | 53 | 59.67 | 2.66 | 1.95 | 2.97, 3.10 | -1.14 | 30, 42 |
| 18 | -6.54 | 54 | 95.23 | 4.21 | 3.08 | 2.81, 2.84, 2.98 | 0.27, 0.24, 0.10 | 26, 42, 27 |
| 19 | 3.74 | 55 | 105.56 | 4.16 | 3.05 | 2.80, 2.84, 2.85 | 0.15, 0.21, 0.20 | 27, 42, 26 |
| 20 | 16.35 | 56 | 116.95 | 5.38 | 3.94 | 3.25 | 0.69 | 30 |
| 21 | -10.39 | 57 | 96.91 | -1.32 | -0.97 | 0.81, 1.18 | -1.78, -2.15 | 43, 26 |
| 22 | -5.96 | 58 | 85.14 | 14.88 | 10.90 | 5.80, 5.98, 6.04 | 5.10, 4.92, 4.86 | 30, 44, 26, 27 |
| 23 | 0.15 | 59 | 99.72 | 6.41 | 4.70 | 6.45 | -1.75 | 45 |
| 24 | -60.32 | 60 | 40.79 | 4.87 | 3.57 | 4.46 | -0.89 | 46 |
| 25 | -57.31 | 61 | 45.33 | 3.34 | 2.45 | 3.52 | -1.07 | 46 |
| 26 | -35.62 | 62 | 66.59 | 3.77 | 2.76 | 3.80 | -1.04 | 46 |
| 27 | -32.56 | 63 | 70.46 | 2.96 | 2.17 | 3.66 | -1.49 | 46 |
| 28 | -37.01 | 64 | 65.71 | 3.26 | 2.39 | 3.70, 3.75 | -1.31, -1.36 | 47, 48 |
| 29 | -50.39 | 65 | 52.23 | 3.36 | 2.46 | 3.18, 3.26 | -0.72, -0.80 | 30, 49 |
| 30 | -24.89 | 66 | 77.75 | 3.34 | 2.45 | 3.18 | -0.73 | 26 |
| 31 | -109.59 | 67 | 5.95 | -9.56 | -7.00 | 3.13, 3.75 | -10.13, -10.75 | 43, 50 |
| 32 | -210.13 | 31 | -109.59 | 5.44 | 3.99 | 4.77 | -0.78 | 51 |
| 33 | -93.52 | 68 | 10.77 | 1.69 | 1.24 | 3.09 | -1.85 | 26 |
| 34 | -102.49 | 69 | -0.15 | 3.64 | 2.67 | 3.35 | -0.68 | 42 |
| 35 | -59.85 | 70 | 42.95 | 3.18 | 2.33 | 3.40 | -1.07 | 51 |
| 36 | 28.93 | 71 | 133.05 | 1.86 | 1.36 | 1.30, 1.36, 1.45 | 0.06, 0.00, -0.09 | 27, 26, 52, 51 |
| H2O | -72.85 | H3O+ | 33.13 | | | | | |