Octopamine Levels in Blattella Germanica L. Tissues by Capillary Gas Chromatography with Electron Capture Detection
Abstract
:Introduction

Results and Discussion
Derivatization conditions and GC-ECD qualitative confirmation
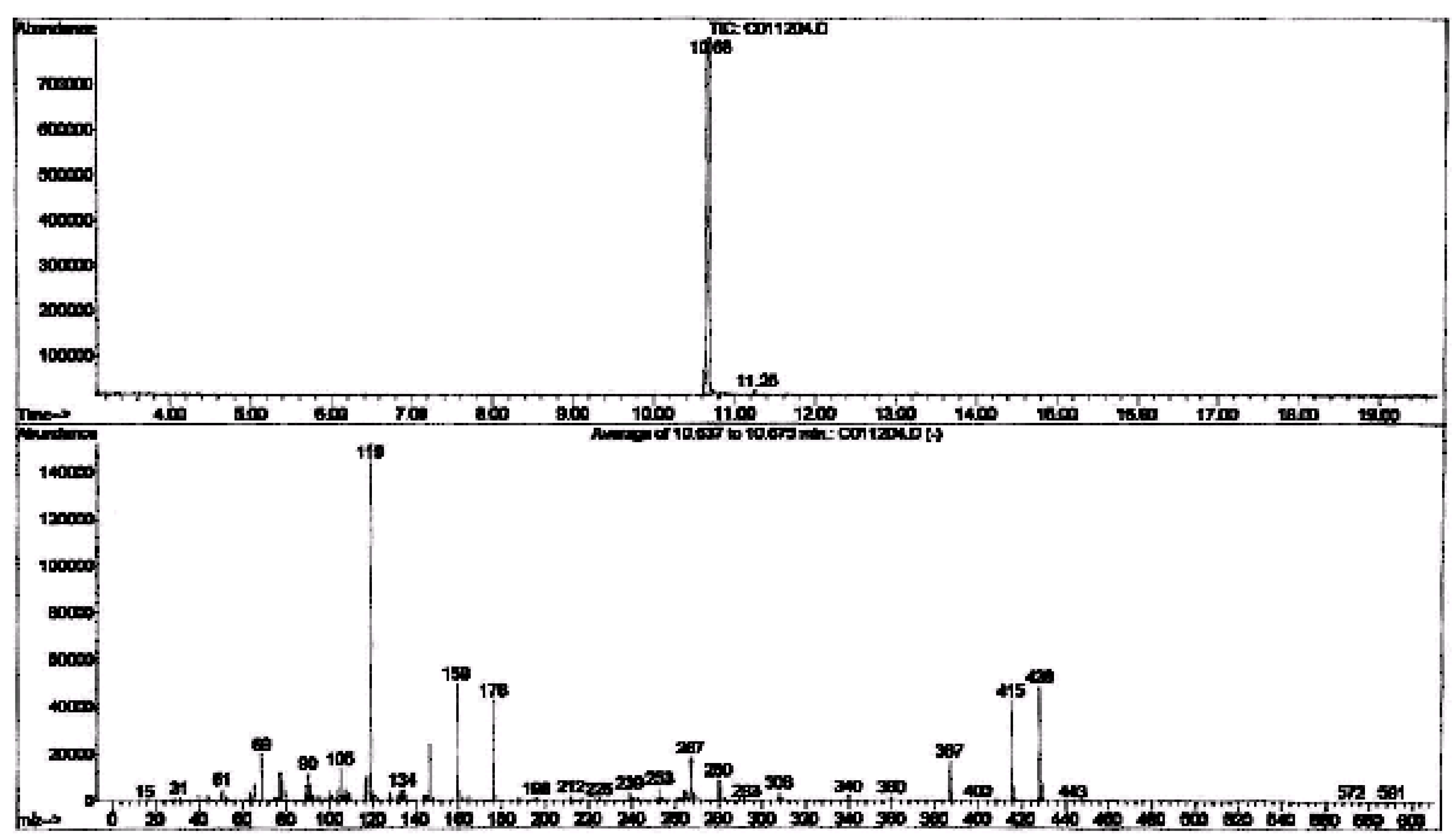
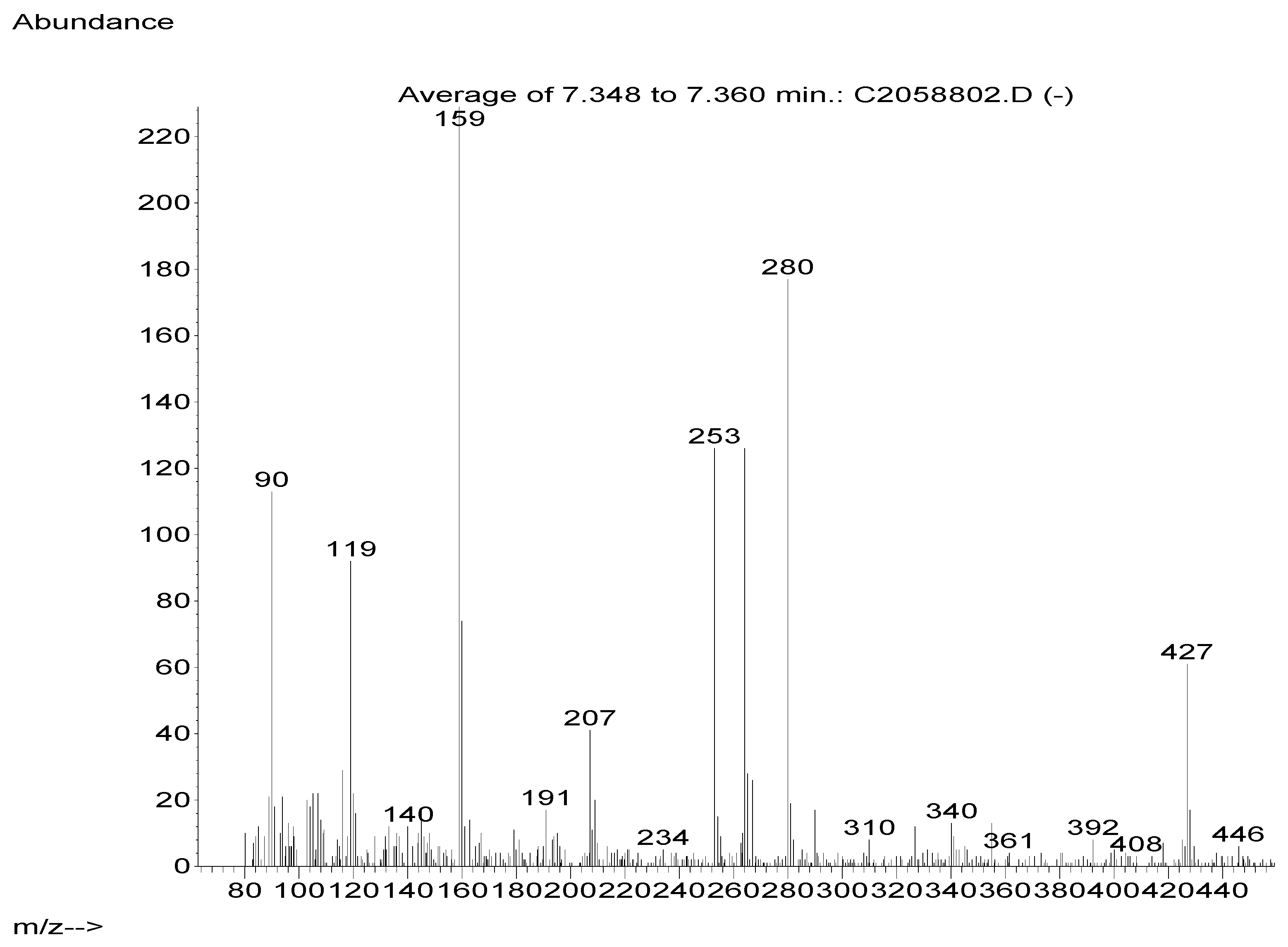
Identification of derivative products by GC-MS
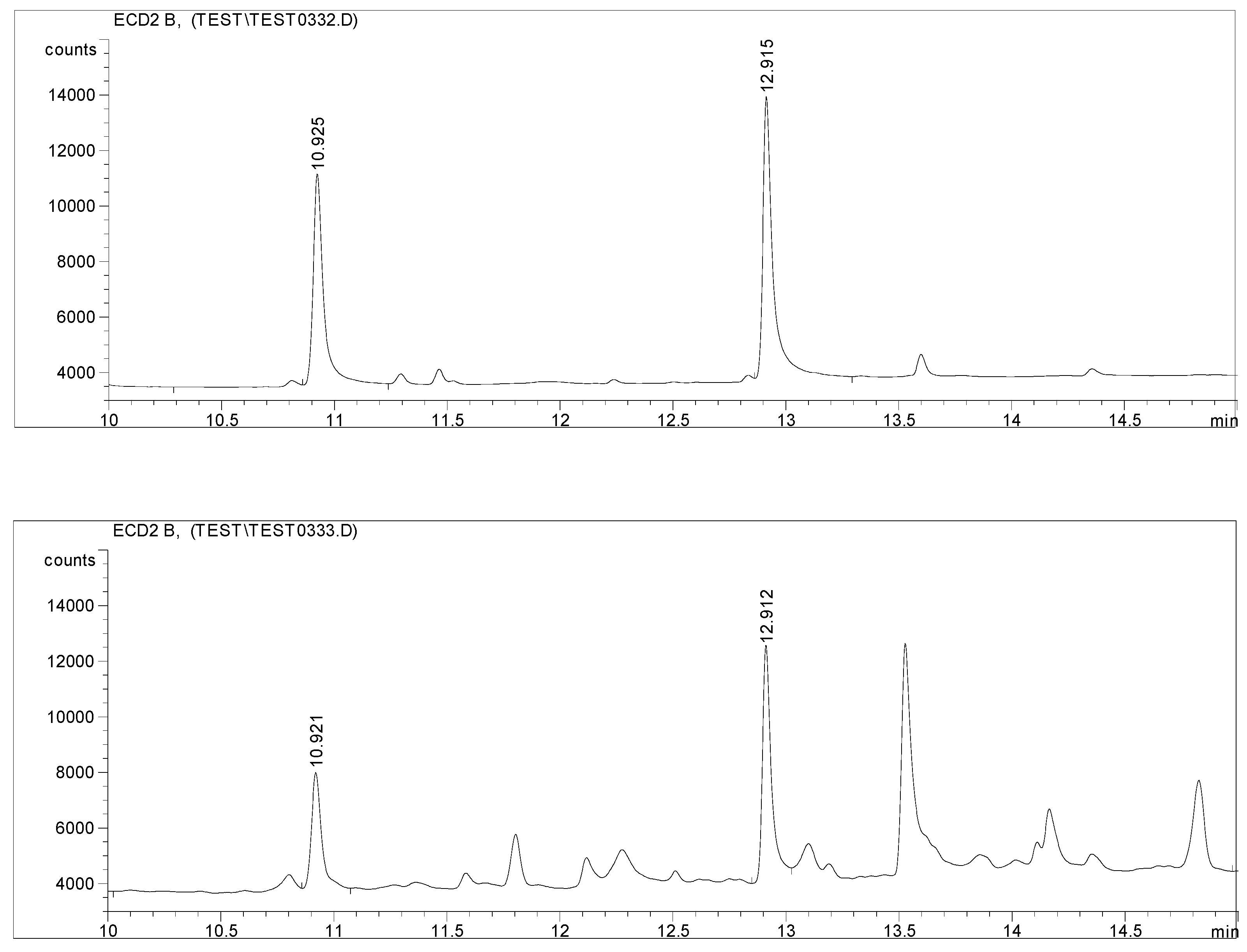
Method validation for GC-ECD
Application of method: OA levels found in homogenizing tissue of Blattella germanica L. under control and various chemical stress conditions
| Chemicals applied | OA level(ng/head)(n=5 *) | Percentage compared to baseline value (%) |
|---|---|---|
| solvent (butanone) | 0.69±0.07 | 100 |
| imidacloprid | 0.85±0.25 | 123 |
| avermectin | 1.09±0.37 | 158 |
| phenyl methyl alcohol | 1.21±0.53 | 175 |
| lindane | 1.27±0.48 | 184 |
| allethrin | 2.03±0.06 | 294 |
| permethrin | 2.28±0.52 | 330 |
| phenyl ethyl alcohol | 3.65±0.75 | 529 |
| BAO50 | 3.97±0.75 | 575 |
| methomyl | 4.52±0.91 | 655 |
| BAO118 | 5.43±0.81 | 787 |
| eugenol | 5.83±1.08 | 845 |
| CDM | 6.75±1.23 | 978 |
| AIO12 | 6.99±0.49 | 1013 |
| trichlorfon | 14.52±2.73 | 2104 |
| chlorfluazuron | 17.33±3.69 | 2512 |
| cinnamic alcohol | 17.47±4.37 | 2532 |
| malathion | 19.48±5.14 | 2823 |
Conclusions
Experimental
Materials and reagents
Sample homogenates
Derivatization
Gas chromatography
GC-ECD
GC-MS
Method validation
Acknowledgements
References
- Roeder, T. Octopamine in invertebrates. Prog. Neurobiol. 1999, 59, 533–561. [Google Scholar] [CrossRef] [PubMed]
- Vanderlinden, C.; Mallefet, J. Synergic effects of tryptamine and octopamine on ophiuroid luminescence (Echinodermata). J. Exp. Biol. 2004, 207, 3749–3756. [Google Scholar] [CrossRef] [PubMed]
- Roeder, T. Tyramine and Octopamine: Modulation at Different Levels. Annu. Rev. Entomol. 2004.
- Rogers, S.M.; Matheson, T.; Sasaki, K.; Kendrick, K.; Simpson, S. J.; Burrows, M. Substantial changes in central nervous system neurotransmitters and neuromodulators accompany phase change in the locust. J. Exp. Biol. 2004, 207, 3603–3617. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Benvenuti, S.; Melegari, M. High-performance liquid chromatography methods for the analysis of adrenergic amines and flavanones in Citrus aurantium L. var. amara. Phytochem. Anal. 2004, 15, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Beltz, B.S. Distribution and functional anatomy of amine-containing neurons in decapod crustaceans. Microsc. Res. Tech. 1999, 44, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Bicker, G. Biogenic amines in the brain of the honeybee: cellular distribution, development, and behavioral functions. Microsc. Res. Tech. 1999, 44, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, A.; Sukhanova, M. J.; Rauschenbach, I. Y. Biogenic amines in Drosophila virilis under stress conditions. Biosci. Biotechnol. Biochem. 2000, 64, 2625–2630. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.P.; Evans, P. D. Stress-induced changes in octopamine levels of insect haemolymph. Insect Biochem. 1984, 14, 135–138. [Google Scholar]
- Goudey-Perriere, F.; Barreteau, H.; Perriere, C.; Gayral, P.; Jacquot, C.; Brousse-Gaury, P. Biogenic amine levels in the cockroach Blaberus craniifer Burm. nervous system. Comp Biochem. Physiol C. 1991, 100, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Goudey-Perriere, F.; Grosclaude, J. M.; Nembo, B.; Barreteau, H.; Jacquot, C.; Gayral, P. Levels of biogenic amines in larvae and adults of the rat hookworm, Nippostrongylus brasiliensis (Nematoda). Comp Biochem. Physiol A Physiol 1997, 118, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Hiripi, L.; Rozsa, K.S. Octopamine- and dopamine-sensitive adenylate cyclase in the brain of Locusta migratoria during its development. Cell Mol. Neurobiol. 1984, 4, 199–206. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, R.G.; Midgley, J. M.; Watson, D.G.; Evans, P.D. Biogenic amines: their occurrence, biosynthesis and metabolism in the locust, Schistocerca gregaria, by gas chromatography-negative-ion chemical ionisation mass spectrometry. J. Chromatogr. 1991, 562, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Nusrat, S.; Midgley, J. M. Analysis of acidic metabolites of biogenic amines in the nervous tissues of the Periplaneta Americana by gas-chromatography negative ion chemical ionization mass spectrometry (GC-NICIMS). Pak. J. Sci. Ind. Res. 1995, 38, 256–260. [Google Scholar]
- Amendola, L.; Molaioni, F.; Botre, F. Detection of beta-blockers in human urine by GC-MS-MS-EI: perspectives for the antidoping control. J. Pharm. Biomed. Anal. 2000, 23, 211–221. [Google Scholar] [CrossRef] [PubMed]
© 2005 by MDPI (http://www.mdpi.org).
Share and Cite
Pandey, C.; Li, W.; Wang, Y.; Jiang, S. Octopamine Levels in Blattella Germanica L. Tissues by Capillary Gas Chromatography with Electron Capture Detection. Int. J. Mol. Sci. 2005, 6, 188-197. https://doi.org/10.3390/i6030188
Pandey C, Li W, Wang Y, Jiang S. Octopamine Levels in Blattella Germanica L. Tissues by Capillary Gas Chromatography with Electron Capture Detection. International Journal of Molecular Sciences. 2005; 6(3):188-197. https://doi.org/10.3390/i6030188
Chicago/Turabian StylePandey, Canping, Weixi Li, Yongshan Wang, and Shuren Jiang. 2005. "Octopamine Levels in Blattella Germanica L. Tissues by Capillary Gas Chromatography with Electron Capture Detection" International Journal of Molecular Sciences 6, no. 3: 188-197. https://doi.org/10.3390/i6030188




