Enzyme Chemiluminescence Immuno Assay of Free hCGβ in Serum Based on Superparamagnetic Polymer Microbeads
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and surface modification of the SPMP microbeads

2.2. Application in the detection of free hCGβ



2.3. Optimization of the operation parameters of the sandwich magnetic ECLIA of free hCGβ



2.4. Sensitivity and recovery rate
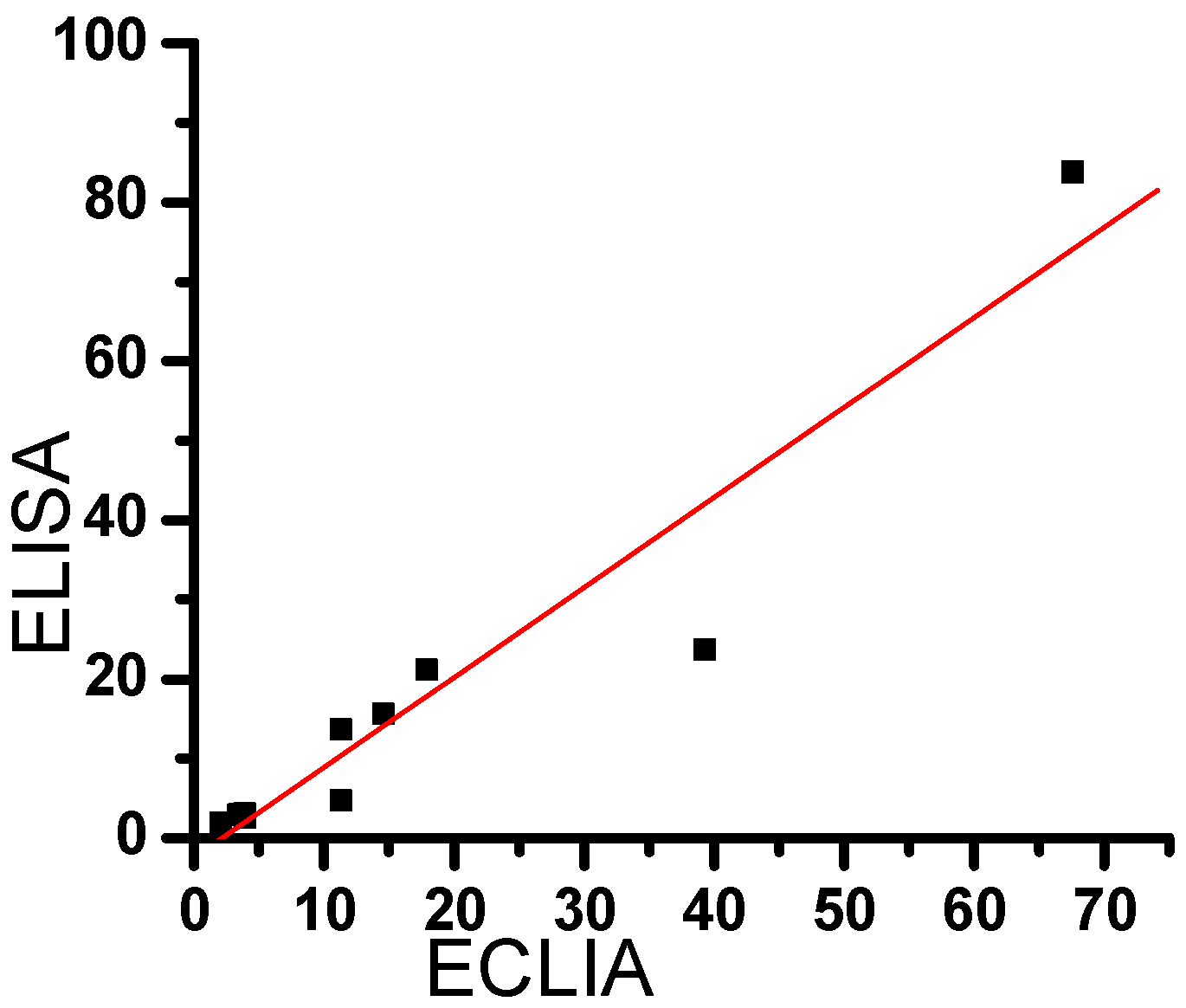
2.5. Comparison between the results of clinical samples, by ECLIA and ELISA.
3. Conclusions
4. Experimental Section
4.1. General
4.2. Preparation of Oleic Acid-coating Fe3O4 Magnetic Fluid (1)
4.3. Synthesis of Poly(MA-DVB) SPMP microspheres (2)
4.4. Surface Modification of Poly(MA-DVB) SPMP microspheres with EDA(3)
4.5. Surface functionalization and activation (4)
4.6. Combination of anti-FITC (5)
4.7. Protocols of the whole assay of ECLIA (6)
4.8. Collection of the clinical serum samples (7)
Acknowledgements
References and Notes
- (a) Baselt, D.R.; Lee, G.L.; Natesan, M.; Metzger, S.W.; Sheehan, P.E.; Colton, R. J. A Biosensor Based on Magnetoresistance Technology. Biosens.Bioelectron. 1998, 13, 731-739; (b) Li, G.; Sun, S.; Wilson, R.J.; White, R. L.; Pourmand, N.; Wang, S. X. Spin Valve Sensors for Ultrasensitive Detection of Superparamagnetic Nanoparticles for Biological Applications. Sensors and Actuators A, 2006, 126, 98-106.
- (a) Kircher, M. F.; Mahmood, U.; King, R. S.; Weissleder, R.; Josephson, L. A Multimodal Nanoparticle for Preoperative Magnetic Resonance Imaging and Intraoperative Optical Brain Tumor Delineation. Cancer Research 2003, 63, 8122-8125; (b) Funovics M.; Montet, X.; Reynolds, F.; Weissleder, R.; Josephson, L. Nanoparticles for the Optical Imaging of Tumor E-selectin. Neoplasia 2005, 7, 904-911.
- Jordan, A.; Scholz, R.; Scholz, R.; Maier-Hauff, K.; Johannsen, M.; Wust, P.; Nadobny, J. Presentation of a New Magnetic Field Therapy System for the Treatment of Human Solid Tumors with Magnetic Fluid Hyperthermia. Journal of Magnetism and Magnetic Materials 2001, 225, 118–126. [Google Scholar] [CrossRef]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical Application of Functionalized Magnetic Nanoparticles. Journal of Bioscience and Bioengineering 2005, 100, 1–11. [Google Scholar] [CrossRef]
- Sun, L.; Zborowski, M.; Chalmers, J.J. Continuous, Flow-through Immunomagnetic Cell Sorting in a Quadrupole Field. Cytometry 1998, 33, 469–475. [Google Scholar] [CrossRef]
- McCloskey, K.E.; Chalmers, J.J.; Zborowski, M. Magnetic Cell Separation: Characterization of Magnetophoretic Mobility. Anal. Chem. 2003, 75, 6868–6874. [Google Scholar] [CrossRef]
- (a) Luxton, R.; Badesha, J.; Kiely, J.; Hawkins, P. Use of External Magnetic Fields To Reduce Reaction Times in an Immunoassay Using Micrometer-Sized Paramagnetic Particles as Labels (Magnetoimmunoassay). Anal. Chem. 2004, 76, 1715-1719; (b) Chou, P.; Chen, S.; Liao, H.; Lin, P.; Her, G.; Lai, A. C.; Chen, J.; Lin, C.; Chen, Y. Nanoprobe-Based Affinity Mass Spectrometry for Selected Protein Profiling in Human Plasma. Anal. Chem. 2005, 77, 5990-5997; (c) Matsunaga, T.; Kawasaki, M.; Yu, X.; Tsujimura, N; Nakamura, N. Chemiluminescence Enzyme Immunoassay Using Bacterial Magnetic Particles. Anal. Chem. 1996, 68, 3551-3554; (d) Tanaka, T.; Matsunaga, T. Fully Automated Chemiluminescence Immunoassay of Insulin Using Antibody-Protein A-Bacterial Magnetic Particle Complexes. Anal. Chem. 2000, 72, 3518-3522.
- Osaka, T.; Matsunaga, T.; Nakanishi, T.; Arakaki, A.; Niwa, D.; Iida, H. Synthesis of Magnetic Nanoparticles and Their Application to Bioassays. Anal. Bioanal. Chem. 2006, 384, 593–600. [Google Scholar] [CrossRef]
- Li, G.; Fan, J.; Gao, Y. Cross-linking the Linear Polymeric Chains in the ATRP Synthesis of Iron Oxide/Polystyrene Core/Shell Nanoparticles. Chem. Mater. 2004, 16, 1835–1837. [Google Scholar] [CrossRef]
- Kim, B.-S.; Qiu, J.-M.; Wang, J.-P.; Taton, T.A. Magnetomicelles: Composite Nanostructures from Magnetic Nanoparticles and Cross-Linked Amphiphilic Block Copolymers. Nano Lett. 2005, 5, 1987–1991. [Google Scholar] [CrossRef]
- Qiu, G.; Wang, Q.; Nie, M. Polypyrrole-Fe3O4 Magnetic Nanocomposite Prepared by Ultrasonic Irradiation. Macromol. Mater. Eng. 2006, 291, 68–74. [Google Scholar] [CrossRef]
- (a) Liu, X.; Guan, Y.; Ma, Z.; Liu, H. Suface Modification and Characterization of Magnetic Polymer Nanospheres Prepared by Miniemulsion Polymerization. Langmuir 2004, 20, 10278-10283; (b) Ma, Z.-Y.; Guan, Y.-P.; Liu, X.-Q.; Liu, H.-Z. Preparation and characterization of micron-sized non-porous magnetic polymer microspheres with immobilized metal affinity ligands by modified suspension polymerization. Journal of Appl. Polym. Sci. 2005, 96, 2174-2180; (c) Liu, X.; Guan, Y.; Shen, R.; Liu, H. Immobilization of Lipase onto Micron-size Magnetic Beads. Journal of Chromatogr. B 2005, 822, 91-97.
- Hotakainen, K.; Ljungberg, B.; Haglund, C.; Nordling, S.; Paju, A.; Stenman, U.H. Expression of the Free Beta-subunit of Human Chorionic Gonadotropin in Renal Cell Carcinoma: Prognostic Study on Tissue and Serum. Int. J. Cancer 2003, 104, 631–635. [Google Scholar] [CrossRef]
- (a) Spencer, K. The Influence of Fetal Sex in Screening for Down Syndrome in the Second Trimester Using AFP and Free β-hCG. Prenat. Diagn. 2000, 20, 648-651; (b) Knight, G.J. ; Palomaki, G.E. ; Neveux, L.M., et al. HCG and the Free β-subunit as Screening Tests for Down Syndrome. Prenat. Diagn. 1998, 18, 235-245; (C) Hedstro, M.J.; Grenman, R.; Ramsay, H. Concentration of Free HCGβ Subunit in Serum as a Prognostic Marker for Squamous-cell Carcinoma of the Oral Cavity and Oropharynx. Int. J. Cancer (Pred. Oncol.) 1999, 84, 525-528 ; (d) Hallahan, T.; Krantz, D.; Orlandi, F.; Rossi, C.; Curcio, P.; Macri, S.; Larsen, J.; Buchanan, P.; Macri, J. First Trimester Biochemical Screening for Down Syndrome: Free Beta HCG versus Intact HCG. Prenat. Diagn. 2000, 20, 785-789.
- (a) Wenstrom, K.D.; Owen, J.; Chu, L. B. Free β-hCG Subunit Versus Intact hCG in Down Syndrome Screening. Obstetrics & Gynecology 1997, 90, 370-374; (b) Caroppo, E.; Niederberger, C.; Iacovazzi, P.A.; Correale, M.; Palagiano, A.; Amato, G.D. Human Chorionic Gonadotropin Free β-subunit in the Human Seminal Plasma: a New Marker for Spermatogenesis? European Journal of Obstetrics & Gynecology and Reproductive Biology 2003, 106, 165-169. (c) Nishimura, R. ; Koizumi, T. ; Yokotani, T. ; Taniguchi, R. ; Morisue, K. ; Yoshimura, M. ; Hiranmoy, D. ; Yamaguchi, S. ; Nakagawa, T. ; Hasegewa, K. ; Yasui, H. Molecular Heterogeneity of hCGβ-related Glycoproteins and the Clinical Relevance in Trophoblastic and Non-trophoblastic Tumors. International Journal of Gynecology & Obstetrics 1998, 60 suppl., S29-S32.
- Hsu, J.-J.; Spencer, K.; Aitken, D.A.; Crossley, J.; Choi, T.; Ozaki, M.; Tazawa, H. Urinary Free Beta HCG, Beta Core Fragment and Total Oestriol as Markers of Down Syndrome in the Second Trimester of Pregnancy. Prenat. Diagn. 1999, 19, 146–158. [Google Scholar] [CrossRef]
- Luppa, P.; Spöttl, G.; Saller, B.; Neumeier, D. Cross – Reactivity of a Commercial Chemiluminescence Immunoassay for Human Chorionic Gonadotropin with the Free Beta – subunit . Journal of Biolumin. Chemilumin. 1992, 7, 195–201. [Google Scholar] [CrossRef]
- (a) Cole, L.A. ; Kellner, L.H. ; Isozaki, T. Comparison of 12 Assays for Detecting hCG and Related Molecules in Urine Samples from Down Syndrome Pregnancies. Prenat. Diagn. 1997, 17, 607-614 ; (b) Lin, J.; Ju, H. Electrochemical and Chemiluminescent Immunosensors for Tumor markers. Biosensors and Bioelectronics 2005, 20, 1461-1470.
- Santandreu, M.; Alegret, S.; Fàbregas, E. Determination of β-HCG Using Amperometric Immunosensors Based on a Conducting Immunocomposite. Analytica Chimica Acta 1999, 396, 181–188. [Google Scholar] [CrossRef]
- (a) Nakamural, N.; Lim, T.-K.; Jeong, J.-M.; Matsunaga, T. Flow Immunoassay for Detection of Human Chorionic Gonadotrophin Using a Cation Exchange Resin Packed Capillary Column. Analytica Chimica Acta 2001, 439, 125-130; (b) Lim, T.-K.; Matsunaga, T. Construction of Electrochemical Flow Immunoassay System Using Capillary Columns and Ferrocene Conjugated Immunoglobulin G for Detection of Human Chorionic Gonadotrophin. Biosensors & Bioelectronics 2001, 16, 1063-1069; (c) Nakamura1,N.; Lim T.-K.; Jeong J.-M.; Matsunaga, T. Flow Immunoassay for Detection of Human Chorionic Gonadotrophin Using a Cation Exchange Resin Packed Capillary Column. Analytica Chimica Acta 2001, 439, 125-130; (d) Lin, J.-M.; Tsuji, A.; Maeda, M. Chemiluminescent Flow Injection Determination of Alkaline Phosphatase and Its Applications to Enzyme Immunoassays. Analytical Chimica Acta 1997, 339, 139-146.
- Wang, Y.; Zhu, R.; He, W. Magnetic Properties of Polystyrene-b-poly(2-hydroxylethylmethacrylate)/metal Hybrids. J Appl. Polym. Sci. 2006, 99, 2314–2319. [Google Scholar] [CrossRef]
- (a) Ma W.-L. ; Zheng W.-L. Molecular Oncology, Chin. Sci. press, 2003, September, Beijing; (b) Yang, C.; Guan, Y.; Xing, J.; Liu, J.; An, Z.; Liu, H. Preparation and Surface Modification of Magnetic PMMA Microspheres. Science in China (Ser.B Chemistry) 2004, 34, 265-269; (c) Liu, X. Q.; Guan, Y. P.; Xing, J. M.; Ma, Z. Y.; Liu, H. Z. Synthesis and Properties of Micro-sized Magnetic Polymer Spheres with Epoxy Groups. Chinese J. Chem. Eng. 2003, 11, 731-735.
- (a) Lee, J.; Senna, M. Preparation of Monodispersed Polystyrene Microspheres Uniformly Coated by Magnetite via Heterogeneous Polymerization. Colloid Polym. Sci. 1995, 273, 76-82; (b) Liu, X. Y.; Ding, X. B.; Zheng, Z. H. Synthesis of Novel Magnetic Polymer Microspheres with Amphiphilic Structure. J Appl. Polym. Sci. 2003, 90, 1879-1884.
- Lu, Y.; Yin, Y.; Mayers, B.T.; Xia, Y. Modifying the Surface Properties of Superparamagnetic Iron Oxide Nanoparticles through a Sol-Gel Approach. Nano Lett. 2002, 2, 183–186. [Google Scholar] [CrossRef]
- Zhao, L.-X.; Lin, J.-M.; Qu, F. Micro-plate Magnetic Chemiluminescence Enzyme Immunoassay for Rapid, Sensitive Determination of Human Chorionic Gonadotropin (hCG). Acta Chimca Sinica 2004, 64, 71–77. [Google Scholar]
- Gillevet, P.M. Chemiluminescent Multiplex DNA Sequencing. Nature 1990, 348, 657–658. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Guo, X.; Guan, Y.; Yang, B.; Wang, Y.; Lan, H.; Shi, W.; Yang, Z.; Lu, Z. Enzyme Chemiluminescence Immuno Assay of Free hCGβ in Serum Based on Superparamagnetic Polymer Microbeads. Int. J. Mol. Sci. 2006, 7, 274-288. https://doi.org/10.3390/i7080274
Guo X, Guan Y, Yang B, Wang Y, Lan H, Shi W, Yang Z, Lu Z. Enzyme Chemiluminescence Immuno Assay of Free hCGβ in Serum Based on Superparamagnetic Polymer Microbeads. International Journal of Molecular Sciences. 2006; 7(8):274-288. https://doi.org/10.3390/i7080274
Chicago/Turabian StyleGuo, Xiaoying, Yueping Guan, Bin Yang, Yongning Wang, Hualong Lan, Wentang Shi, Zhenghui Yang, and Zuhong Lu. 2006. "Enzyme Chemiluminescence Immuno Assay of Free hCGβ in Serum Based on Superparamagnetic Polymer Microbeads" International Journal of Molecular Sciences 7, no. 8: 274-288. https://doi.org/10.3390/i7080274



