Variation of the Nuclear, Subnuclear and Chromosomal Flavanol Deposition in Hemlock and Rye
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Tsuga (histology)
2.1.1.1 Nuclei with different degrees and modes of flavanol deposition
2.1.1.2 Chromosomes
2.1.1.3 Conifers with very small nucleoli
2.1.2. Secale cereale (histology)
2.1.2.1 Nuclei of rootlets at interphase
2.1.2.2 Mitotic nuclei
2.1.2.3 Elongation zone and root cap
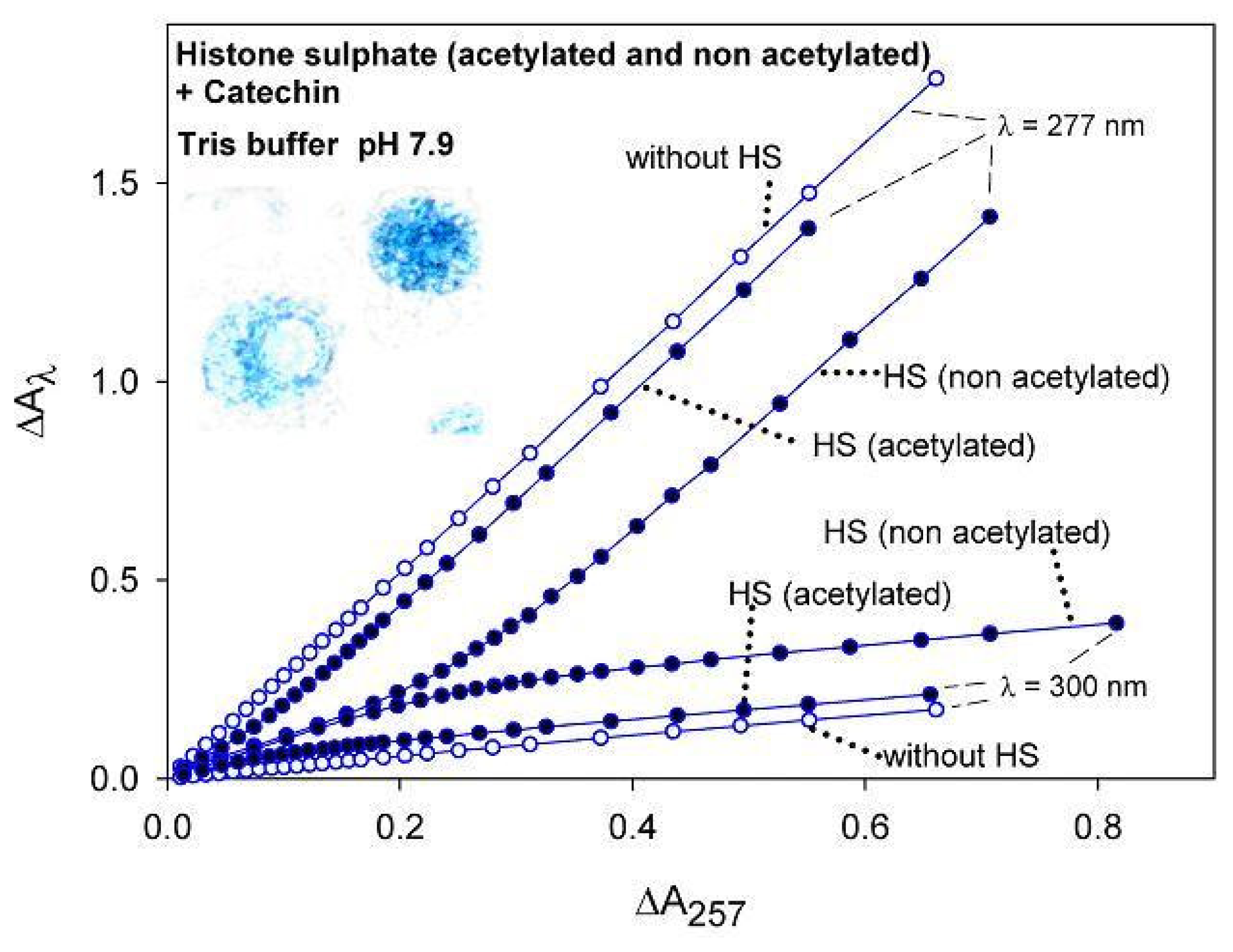
2.1.3. UV-VIS spectroscopic-kinetic analysis and titrations
2.2. Discussion
2.3. Conclusions
3. Experimental Section
3.1. Plant-Material
3.2. Histology
3.3. Chemicals (for Titration, Kinetics and Acetylation)
3.4. Buffers and Stock solutions (for Titration, Kinetics and Acetylation)
3.5. Acetylation of histones
3.6. UV-VIS spectroscopic titrations
3.7. UV-VIS spectroscopic-kinetic analysis: reactions of 1-acetylimidazole in different buffers



| Half time | Tris | imidazole | phosphate |
|---|---|---|---|
| t ½ [min] | 3.7 | 37.6 | 20.8 |
References and Notes
- Polster, J.; Dithmar, H.; Burgemeister, R; Friedemann, G.; Feucht, W. Flavonoids in plant nuclei: detection by laser microdissection and pressure catapulting (LMPC), in vivo staining, UV-visible spectroscopic titration. Physiologia Plantarum 2006, 128, 163–174. [Google Scholar]
- Feucht, W.; Treutter, D.; Polster, J. Flavanol binding of nuclei from tree species. Plant Cell Reports 2004, 22, 430–436. [Google Scholar]
- Polster, J. Chromatography, Titration, and Reaction Kinetics: UV-VIS Spectroscopic Evaluation on the Basis of the Mauser Diagrams; Research Signpost: Trivandrum, Kerala, India, 2006. [Google Scholar]
- Polster, J.; Dithmar, H.; Feucht, W. Are histones the targets for flavan-3-ols (catechins) in nuclei? Biol. Chem. 2003, 384, 997–1006. [Google Scholar]
- Saslowski, D.; Winkel-Shirley, B. Localization of flavonoid enzymes in Arabidopsis roots. Plant J 2001, 27, 37–48. [Google Scholar]
- Hutzler, P.; Fischbach, R.; Heller, W.; Jungblut, T.P.; Reuber, S.; Schmitz, R.; Veit, M.; Weissenböck, G.; Schnitzler, J.P. Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. J. Exp. Bot 1998, 49, 953–965. [Google Scholar]
- Ahmed, C.M.; Padayatty, J.D. Presence and function of a free pool of histones in the nucleosol of rice embryos. Indian J. Biochem. Biophys 1982, 19, 155–159. [Google Scholar]
- Covelo, G.; Sarandeses, C.S.; Díaz-Jullien, C.; Freire, M. Prothymosin α interacts with free core histones in the nucleus of dividing cells. Journal of Biochemistry 2006, 140(5), 627–637. [Google Scholar]
- Gunjan, A.; Paik, J.; Verreault, A. Regulation of histone synthesis and nucleosome assembly. Biochimie 2005, 87, 625–635. [Google Scholar]
- Tanaka, T.; Kusano, R.; Kondou, K.; Kawashiri, M.; Kouno, I. Reaction of Flavan-3-ol-aldehyde condensation products with amino acids and thiol compounds. Third Tannin Conference, Bend, Oregon (USA), Juli 20–25; 1998. [Google Scholar]
- Haslam, E. Practical polyphenolics. From structure to molecular recognition and physiological action; Cambridge; University Press, 1998. [Google Scholar]
- Loidl, P. A plant dialect of the histone language. Trends in Plant Science 2004, 9(2), 84–90. [Google Scholar]
- Berger, S.; Dithmar, H.; Polster, J.; Feucht, W. Are there controlled interactions between histones and epigallocatechin gallate. Free Radical Research 2003, 37 Supplement 2, 29–30. [Google Scholar]
- Mauser, H. Formale Kinetik; Bertelsmann Universitätsverlag: Düsseldorf, 1974. [Google Scholar]
- Hrazdina, G.; Jensen, R.A. Spatial organization of enzymes in plant metabolic pathways. Ann. Rev. Plant Physiol. Plant Mol. Biol 1992, 43, 241–267. [Google Scholar]
- Pfeiffer, J.; Kühnel, C.; Brandt, J.; Duy, D.; Punyasiri, P.A.N.; Forkmann, G.; Fischer, T.C. Biosynthesis of flavan-3-ols by leucoanthocyanidin 4-reductases and anthocyanidin reductases in leaves of grape (Vitis vinifera L.) apple (Malus x domestica Borkh.) and other crops. Plant Physiol. and Biochemistry 2006, 44, 323–334. [Google Scholar]
- Margueron, R.; Trojer, P.; Reinberg, D. The key to development: interpreting the histone code? Current Opinion in Genetics and Development 2005, 15, 163–176. [Google Scholar]
- Probst, A.V.; Fransz, P.F.; Pazkowski, J.; Mittelsten-Scheid, O. Two means of transcriptional reactivation with heterochromatin. Plant J 2003, 33, 743–749. [Google Scholar]
- Fuchs, J.; Demidov, D.; Houben, A.; Schubert, J. Chromosomal histone modification patterns – from conservation to diversity. Trends in Plant Sci 2006, 11, 199–208. [Google Scholar]
- Godin, D.E.; Stack, S.M. Heterochromatic connections between the chromosomes of Secale cereale. Can. J. Genet. Cytol 1975, 17, 269–273. [Google Scholar]
- Bedbrook, J.R.; Jones, J.; O’Dell, M.; Thompson, R.D.; Flavell, R.B. A molecular description of telomeric heterochromatin in Secale species. Cell 1980, 19(2), 545–560. [Google Scholar]
- Gustafson, J.P.; Lukaszewski, A.L.; Bennet, M.D. Somatic deletion and redistribution of telomeric heterochromatin in the genus Secale and in Triticale. Chromosoma 1983, 88, 293–298. [Google Scholar]
- De Putter, M.; Van de Vooren, J.G. Identification of Allium cepa cultivars by means of statistical analysis of C-banded chromosomes. Euphytica 1988, 39, 153–160. [Google Scholar]
- Robinson, P.J.J.; Rhodes, D. Structure of the ‘30 nm’ chromatin fibre: A key role for the linker histone. Curr. Opinion in Structural Biol 2006, 16, 336–343. [Google Scholar]
- Anderson, J.S.; Lam, Y.W.; Leung, A.K.L.; Ong, S.; Lyon, C.E.; Lamond, A.L.; Mann, M. Nuclear proteome dynamics. Nature 2005, 433, 77–83. [Google Scholar]
- Mineur, P.; Jennane, A.; Thiry, M.; Deltour, R.; Goessens, G. Ultrastructural distribution of DNA within plant meristematic cell nucleoli during activation and the subsequent inactivation by a cold stress. J. Struct. Biol 1998, 123, 199–210. [Google Scholar]
- Feucht, W.; Dithmar, H.; Polster, J. Nuclei of tea flowers as targets for flavanols. Plant Biology 2004, 6, 696–701. [Google Scholar]
- Feucht, W.; Treutter, D.; Dithmar, H.; Polster, J. Flavanols in somatic cell division and male meiosis of tea (Camellia sinensis) anthers. Plant Biology 2005, 7, 168–175. [Google Scholar]
- Barckhausen, R. Ultrastructural changes in wounded plant storage tissue cells. In Biochemistry of wounded plant tissues; Kahl, G., Ed.; Walter de Gruyter & Co: Berlin New York, 1978; pp. 1–42. [Google Scholar]
- Chua, Y.L.; Brown, A.P.C.; Gray, J.C. Targeted histone acetylation and altered nuclease accessibility over short regions of the pea plastocyan gene. The Plant Cell 2001, 13, 599–612. [Google Scholar]
- Thies, M.; Fischer, R. Über eine neue Farbreaktion zum mikrochemischen Nachweis und zur quantitativen Bestimmung von Catechinen. Microchim. Acta (Wien) 1971, 13, 9–13. [Google Scholar]
- Feucht, W.; Nachit, M. Flavolans and growth-promoting catechins in young shoot tips of Prunus species and hybrids. Physiol. Plant 1977, 40, 230–234. [Google Scholar]
- Coleman, A.W.; Maguire, M.J.; Coleman, J.R. Mithramycin- and 4,6-diamidino-2-phenylindole (DAPI)-DNA staining for fluorescence microspectro-photometric measurement of DNA in nuclei, plastids and virus particles. J. Histochem. Cytochem 1981, 29, 959–968. [Google Scholar]
- Hudson, B.; Upholt, W.B.; Devinny, J.; Vinograd, J. The use of an ethidium analogue in the dyebuoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc. Natl. Acad. Sci. U.S.A 1969, 62, 813–820. [Google Scholar]
- Gerlach, D. Botanische Mikrotechnik: Eine Einführung; Georg Thieme Verlag: Stuttgart, 1969. [Google Scholar]
- Polster, J.; Lachmann, H. Spectrometric Titrations. Analysis of Chemical Equilibria; VCH Verlagsgesellschaft mbH: Weinheim, 1989. [Google Scholar]
© 2007 by MDPI ( http://www.mdpi.org) Reproduction is permitted for noncommercial purposes.
Share and Cite
Feucht, W.; Dithmar, H.; Polster, J. Variation of the Nuclear, Subnuclear and Chromosomal Flavanol Deposition in Hemlock and Rye. Int. J. Mol. Sci. 2007, 8, 635-650. https://doi.org/10.3390/i8070635
Feucht W, Dithmar H, Polster J. Variation of the Nuclear, Subnuclear and Chromosomal Flavanol Deposition in Hemlock and Rye. International Journal of Molecular Sciences. 2007; 8(7):635-650. https://doi.org/10.3390/i8070635
Chicago/Turabian StyleFeucht, Walter, Heike Dithmar, and Jürgen Polster. 2007. "Variation of the Nuclear, Subnuclear and Chromosomal Flavanol Deposition in Hemlock and Rye" International Journal of Molecular Sciences 8, no. 7: 635-650. https://doi.org/10.3390/i8070635




