Accelerator Analysis of Tributyltin Adsorbed onto the Surface of a Tributyltin Resistant Marine Pseudoalteromonas sp. Cell
Abstract
:1. Introduction
2. Results and Discussion
2.1. Accelerator Analysis of the Sn Atoms Originated in TBT, or SnCl2, Adsorbed by Pseudoalteromonas sp. TBT1 Cells
2.2. Estimation of Adsorption State of TBT Based on the Accelerator Analytical Results
3. Experimental Section
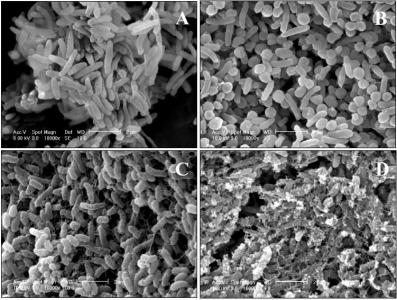
3.1. Isolation of the Pseudoalteromonas sp. TBT1 Strain
3.2. Preparation of Resting Cells of the Isolate, Pseudoalteromonas sp. TBT1
3.3. Preparation of the Cells Treated with Lysozyme, Heat, Ethanol Exposure, and Acidic Exposure
3.4. Enumeration of the Number of Colony-forming Cells
3.5. Accelerator Analysis
3.6. Preparation of Samples for Scanning Electron Microscopy
3.7. Reagents
4. Conclusions
References and Notes
- Guruge, KS; Tanabe, S. Contamination by Persistent Organochlorines and Butyltin Compounds in the West Coast of Sri Lanka. Mar. Pollut. Bull. 2001, 42, 179–186. [Google Scholar]
- Nemanic, TM; Leskovsek, H; Horvat, M; Vriser, B; Bolje, A. Organotin Compounds in the Marine Environment of the Bay of Piran, Northern Adriatic Sea. J. Environ. Monit. 2002, 4, 426–430. [Google Scholar]
- Sudaryanto, A; Takahashi, S; Iwata, H; Tanabe, S; Ismail, A. Contamination of Butyltin Compounds in Malaysian Marine Environments. Environ. Pollut. 2004, 130, 347–358. [Google Scholar]
- Shim, WJ; Hong, SH; Kim, NS; Yim, UH; Li, D; Oh, JR. Assessment of Butyl- and Phenyltin Pollution in the Coastal Environment of Korea using Mussels and Oysters. Mar. Pollut. Bull. 2005, 51, 922–931. [Google Scholar]
- Murai, R; Takahashi, S; Tanabe, S; Takeuchi, I. Status of Butyltin Pollution along the Coasts of Western Japan in 2001, 11 Years after Partial Restrictions on the Usage of Tributyltin. Mar. Pollut. Bull. 2005, 51, 940–949. [Google Scholar]
- Bhosle, NB; Garg, A; Harji, R; Jadhav, S; Sawant, SS; Krishnamurthy, V; Anil, C. Butyltins in the Sediments of Kochi and Mumbai Harbors, West Coast of India. Environ. Int. 2006, 32, 252–258. [Google Scholar]
- Antizar-Ladislao, B. Environmental Levels, Toxicity and Human Exposure to Tributyltin (TBT)-Contaminated Marine Environment. A Review. Environ. Int. 2008, 34, 292–308. [Google Scholar]
- Weidenhaupt, A; Arnold, C; Müller, SR; Haderlein, SB; Schwarzenbach, RP. Sorption of Organotin Biocides to Mineral Surfaces. Environ. Sci. Technol. 1997, 31, 2603–2609. [Google Scholar]
- Hoch, M. Organotin Compounds in the Environment–an Overview. Appl. Geochem. 2001, 16, 719–743. [Google Scholar]
- Hoch, M; Alonso-Azcarate, J; Lischick, M. Assessment of Adsorption Behavior of Dibutyltin (DBT) to Clay-Rich Sediments in Comparison to the Highly Toxic Tributyltin (TBT). Environ. Pollut. 2003, 123, 217–227. [Google Scholar]
- Chien, L-C; Hung, T-C; Choang, K-Y; Yeh, C-Y; Meng, P-J; Shieh, M-J; Han, B-C. Daily Intake of TBT, Cu, Zn, Cd and As for Fishermen in Taiwan. Sci. Total Environ. 2002, 285, 177–185. [Google Scholar]
- Ueno, D; Inoue, S; Takahashi, S; Ikeda, K; Tanaka, H; Subramanian, AN; Fillmann, G; Lam, PK; Zheng, J; Muchtar, M; Prudente, M; Chung, K; Tanabe, S. Global Pollution Monitoring of Butyltin Compounds using Skipjack Tuna as a Bioindicator. Environ. Pollut. 2004, 127, 1–12. [Google Scholar]
- Horiguchi, T; Kojima, M; Kaya, M; Matsuo, T; Shiraishi, H; Morita, M; Adachi, Y. Tributyltin and Triphenyltin Induce Spermatogenesis in Ovary of Female Abalone, Haliotis gigantea. Mar. Environ. Res. 2002, 54, 679–684. [Google Scholar]
- Horiguchi, T. Masculinization of Female Gastropod Mollusks Induced by Organotin Compounds, Focusing on Mechanism of Actions of Tributyltin and Triphenyltin for Development of Imposex. Environ. Sci. 2006, 13, 77–87. [Google Scholar]
- Boyer, IJ. Toxicity of Dibutyltin, Tributyltin and Other Organotin Compounds to Humans and to Experimental Animals. Toxicology 1989, 55, 253–298. [Google Scholar]
- Grondin, M; Marion, M; Denizeau, F; Averill-Bates, DA. Tributyltin Induces Apoptotic Signaling in Hepatocytes through Pathways Involving the Endoplasmic Reticulum and Mitochondria. Toxicol. Appl. Pharmacol. 2007, 222, 57–68. [Google Scholar]
- Nakatsu, Y; Kotake, Y; Ohta, S. Tributyltin-Induced Cell Death Is Mediated by Calpain in PC12 Cells. Neurotoxicology 2006, 27, 587–593. [Google Scholar]
- Nakatsu, Y; Kotake, Y; Ohta, S. Concentration Dependence of the Mechanisms of Tributyltin-Induced Apoptosis. Toxicol. Sci. 2007, 97, 438–447. [Google Scholar]
- Hamasaki, T; Sato, T; Nagase, H; Kito, H. The Mutagenicity of Organotin Compounds as Environmental Pollutants. Mutat. Res. 1993, 300, 265–271. [Google Scholar]
- International Maritime Organization. International Convention on the Control of Harmful Anti-Fouling Systems on Ships. 2001.
- Lewis, PN; Riddle, MJ; Hewitt, CL. Management of Exogenous Threats to Antarctica and the Sub-Antarctic Islands: Balancing Risks from TBT and Non-Indigenous Marine Organisms. Mar. Pollut. Bull. 2004, 49, 999–1005. [Google Scholar]
- International Maritime Organization, International Convention for the Control and Management of Ships' Ballast Water and Sediments. 2004.
- Hua, J; Liu, S-M. Butyltin in Ballast Water of Merchant Ships. Ocean Eng. 2007, 34, 1901–1907. [Google Scholar]
- Armstrong, E; Boyd, KG; Burgess, JG. Prevention of Marine Biofouling Using Natural Compounds from Marine Organisms. Biotechnol. Annu. Rev. 2000, 6, 221–241. [Google Scholar]
- Fukagawa, T; Suzuki, S; Fukunaga, K; Suzuki, T; Takama, K. Isolation and Characterization of Tributyltin Chloride-Resistant Marine Vibrio. FEMS Microbiol. Lett. 1992, 93, 83–86. [Google Scholar]
- Fukagawa, T; Suzuki, S. Cloning of Gene Responsible for Tributyltin Chloride (TBTCl) Resistance in TBTCl-Resistant Marine Bacterium, Alteromonas sp. M-1. Biochem. Biophys. Res. Commun. 1993, 194, 733–740. [Google Scholar]
- White, JS; Tobin, JM; Cooney, JJ. Organotin Compounds and their Interactions with Microorganisms. Can. J. Microbiol. 1999, 45, 541–554. [Google Scholar]
- Jude, F; Arpin, C; Brachet-Castang, C; Capdepuy, M; Caumette, P; Quentin, C. TbtABM, a Multidrug Efflux Pump Associated with Tributyltin Resistance in Pseudomonas stutzeri. FEMS Microbiol. Lett. 7–14.
- Cruz, A; Caetano, T; Suzuki, S; Mendo, S. Aeromonas veronii, a Tributyltin (TBT)-Degrading Bacterium Isolated from an Estuarine Environment, Ria de Aveiro in Portugal. Mar. Environ. Res. 2007, 64, 639–650. [Google Scholar]
- Mimura, H; Katakura, R; Ishida, H. Changes of Microbial Populations in a Ship’s Ballast Water and Sediments on a Voyage from Japan to Qatar. Mar. Pollut. Bull. 2005, 50, 751–757. [Google Scholar]
- Mimura, H; Sato, R; Furuyama, Y; Taniike, A; Yagi, M; Yoshida, K; Kitamura, A. Adsorption of Tributyltin by Tributyltin Resistant Marine Pseudoalteromonas sp. cells. Mar. Pollut. Bull. 2008, 57, 877–882. [Google Scholar]
- Kubota, N; Mimura, H; Yamauchi, T; Kitamura, A. Accelerator Analyses of Tributyltin Chloride Associated with a Tributyltin Resistant Marine Microorganism. Mar. Pollut. Bull. 2004, 48, 800–805. [Google Scholar]
- Morisaki, H; Nagai, S; Ohshima, H; Ikemoto, E; Kogure, K. The Effect of Motility and Cell-surface Polymers on Bacterial Attachment. Microbiology 1999, 145, 2797–2802. [Google Scholar]
- Hoch, M; Weerasooriya, R. New Model Calculations of pH-Depending Tributyltin Adsorption onto Montmorillonite Surface and Montmorillonite-Rich Sediment. Environ. Sci. Technol. 2005, 39, 844–849. [Google Scholar]
- Osmekhin, S; Caló, A; Kisand, V; Nõmmiste, E; Kotilainen, H; Aksela, H; Aksela, S. Fragmentation of Molecular Tributyltin Chloride. Int. J. Mass Spectrom. 2008, 273, 48–52. [Google Scholar]
- Hermosin, MC; Martin, P; Cornejo, J. Adsorption Mechanisms of Monobutyltin in Clay Minerals. Environ. Sci. Technol. 1993, 27, 2606–2611. [Google Scholar]






| TBTCl (mM) | Number of Sn, NSn (Sn cell−1)a | Virtual area, So (nm2)b |
|---|---|---|
| 0.0030 | 105.4 | 9.5 |
| 0.010 | 105.9 | 2.9 |
| 0.030 | 106.4 | 0.96 |
| 0.10 | 106.9 | 0.29 |
| 0.15 | 107.1 | 0.20 |
| 0.30 | 107.4 | 0.10 |
| 0.50 | 107.6 | 0.064 |
| 1.0 | 107.8 | 0.035 |
| 3.0 | 108.2 | 0.017 |
| 10 | 108.3 | 0.013 |
© 2008 by MDPI This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mimura, H.; Sato, R.; Sasaki, Y.; Furuyama, Y.; Taniike, A.; Yoshida, K.; Kitamura, A. Accelerator Analysis of Tributyltin Adsorbed onto the Surface of a Tributyltin Resistant Marine Pseudoalteromonas sp. Cell. Int. J. Mol. Sci. 2008, 9, 1989-2002. https://doi.org/10.3390/ijms9101989
Mimura H, Sato R, Sasaki Y, Furuyama Y, Taniike A, Yoshida K, Kitamura A. Accelerator Analysis of Tributyltin Adsorbed onto the Surface of a Tributyltin Resistant Marine Pseudoalteromonas sp. Cell. International Journal of Molecular Sciences. 2008; 9(10):1989-2002. https://doi.org/10.3390/ijms9101989
Chicago/Turabian StyleMimura, Haruo, Ryusei Sato, Yu Sasaki, Yuichi Furuyama, Akira Taniike, Kazutoshi Yoshida, and Akira Kitamura. 2008. "Accelerator Analysis of Tributyltin Adsorbed onto the Surface of a Tributyltin Resistant Marine Pseudoalteromonas sp. Cell" International Journal of Molecular Sciences 9, no. 10: 1989-2002. https://doi.org/10.3390/ijms9101989