Synthesis and Properties of Poly[p-(2,5-dihydroxy)- phenylenebenzobisoxazole] Fiber
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Measurements
2.3. Synthesis
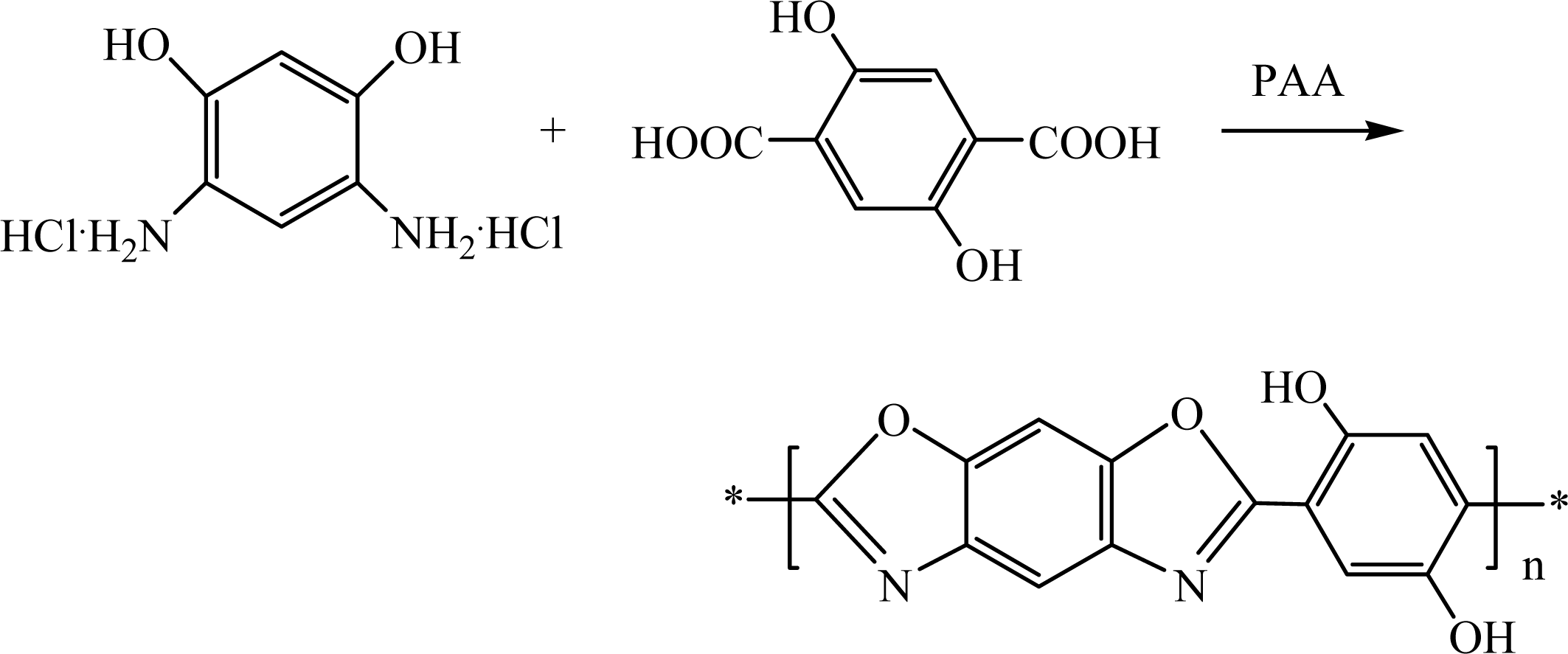
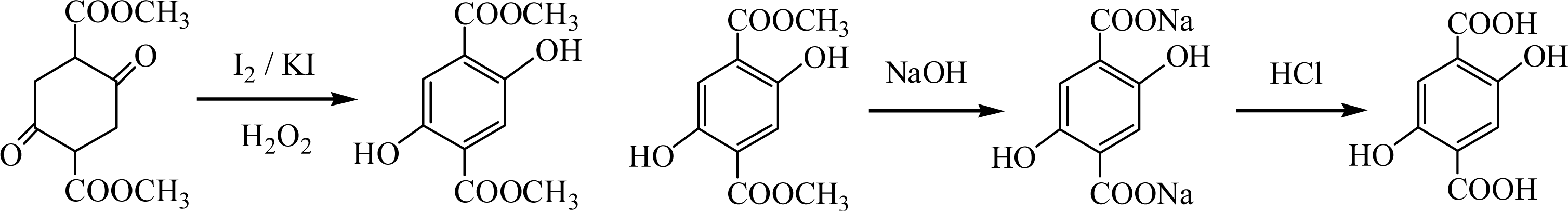
2.3.1. Synthesis of 2,5-Dihydroxyterephthalic acid (DHTA) (Scheme 1)
2.3.2. Polymerization
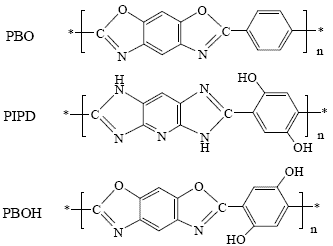
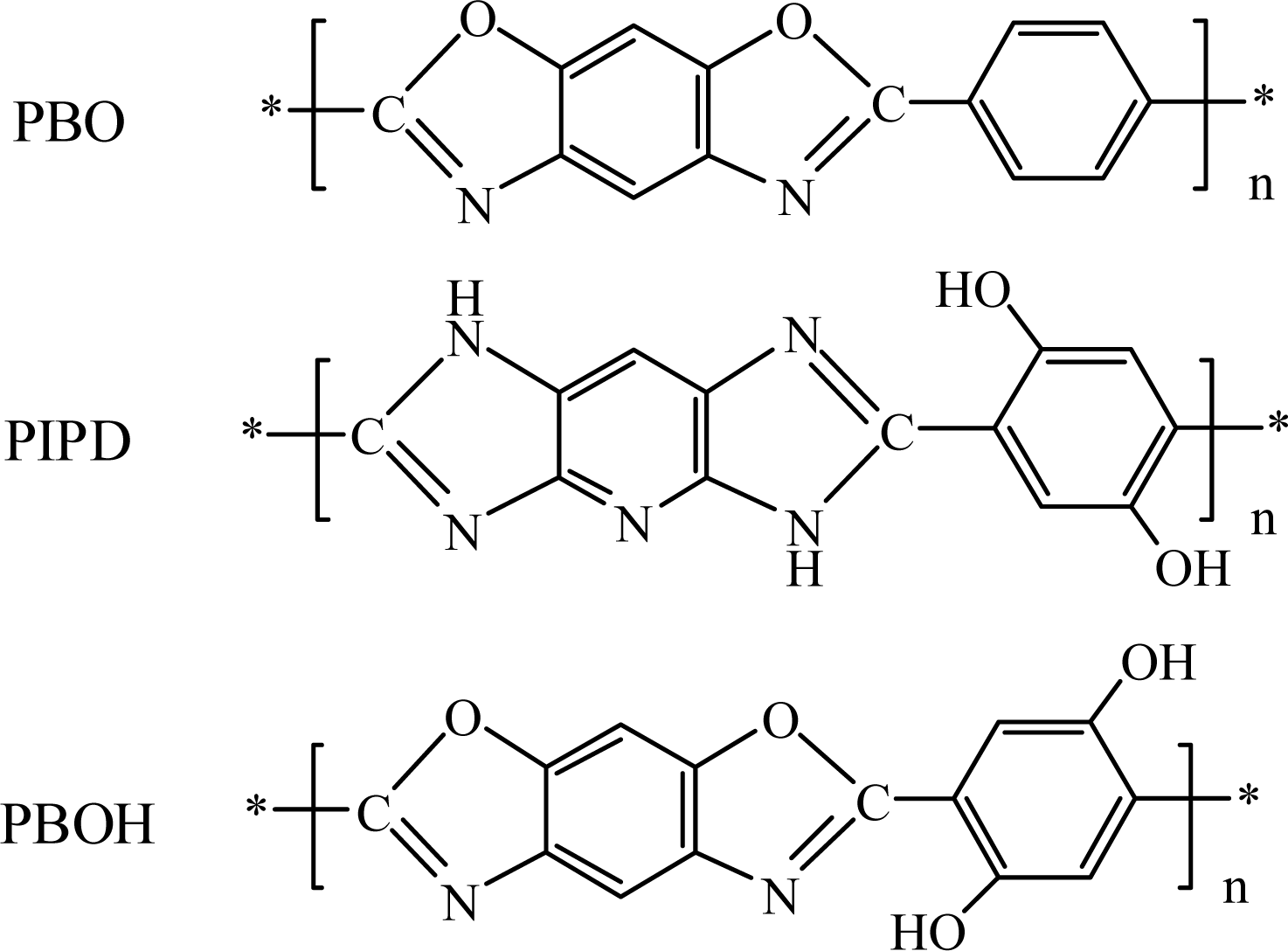
3. Results and Discussion
3.1. Characteristics of the synthesized polymer
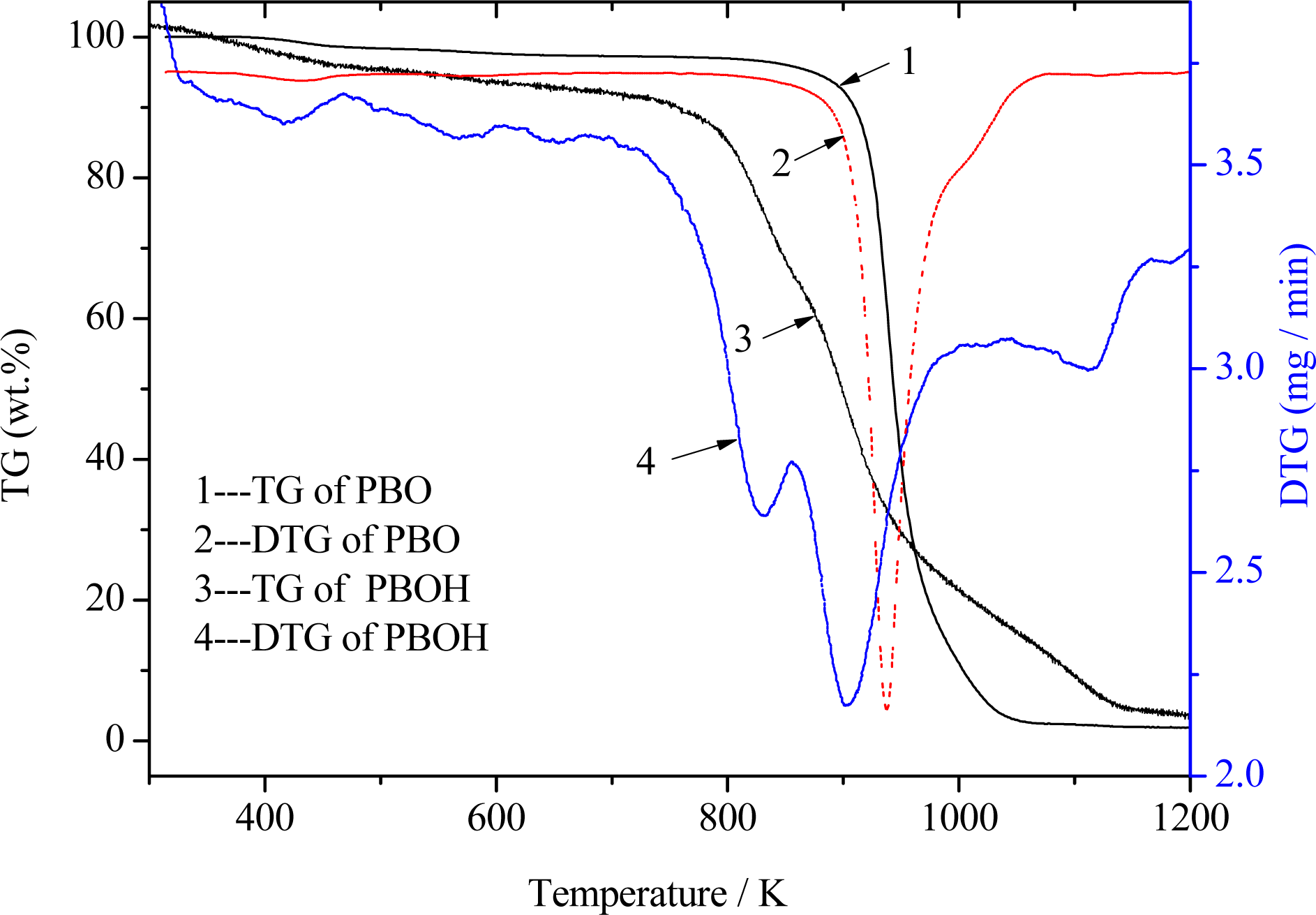
3.2. Thermal decomposition behaviour of PBOH fiber
3.3. XRD and Raman analysis of PBOH fibers
3.4. Mechanical properties of PBOH fibers
3.5. Molecular simulation of PBOH
4. Conclusions
Acknowledgments
References
- Wolfe, J. F. Rigid-rod Polymer Synthesis: Development of Mesophase Polymerization in Strong Acid Solutions. Rec. Symp. Proc 1989, 134, 83–93. [Google Scholar]
- Martin, DC; Thomas, EL. Ultrastructure of Poly(p-phenylenebenzobisoxazole) Fibers. Macromolecules 1991, 24, 2450–2460. [Google Scholar]
- Hu, XD; Shawn, EJ; Byung, GM; Malcolm, B; Polk, SK. Rigid-Rod Polymers: Synthesis, Processing, Simulation, Structure, and Properties. Macromol Mater. Eng 2003, 288, 823–834. [Google Scholar]
- So, YH. Rigid-rod Polymer with Enhanced Lateral Interaction. Progr. Polym. Sci 2000, 25, 137–157. [Google Scholar]
- Bonk, P; Harris, W; Im, JH; Madison, NL; Rosenberg, S. PBX Fiber Mechanical Properties. AFWAL-TR-88-4131. . Wright-Patterson Air Force Base: 1988. [Google Scholar]
- So, YH; Sen, A; Kim, P. Molecular Composite Fibers from Rigid Rod Polymers and Thermoset Resin Matrixes. J. Polym. Sci. A: Polym. Chem 1995, 33, 2893–2899. [Google Scholar]
- McGarry, FJ; Moalli, JE. Mechanical Behaviour of Rigid-rod Polymer Fibers: 2. Improvement of Compressive Strength. Polymer 1991, 32, 1816–1820. [Google Scholar]
- Sikkema, D. J. Design, Synthesis and Properties of a Novel Rigid Rod Polymer, PIPD or 'M5': High Modulus and Tenacity Fibres with Substantial Compressive Strength. Polymer 1998, 24, 5981–5986. [Google Scholar]
- DeTeresa, SJ; Porter, RS; Farris, RJ. A Model For The Compressive Buckling Of Extended Chain Polymers. J. Mater. Sci 1988, 23, 1886–1894. [Google Scholar]
- So, YH; Bell, B; Heeschen, JP; Nyquist, RA; Murlick, CL. Poly (p-Phenylene-benzobisoxazole) Fiber with Polyphenylene Sulfide Pendent Groups. J. Polym. Sci. A: Polym. Chem 1995, 33, 159–164. [Google Scholar]
- Song, YJ; Huang, YD; Lin, H; Wang, Z; Li, J. Study on synthesis and properties of poly(p-phenylene-benzobisoxazole) (PBO) fiber. J. Solid Rocket Technol. (Chinese) 2006, 29, 367–371. [Google Scholar]
- Allen, S. R. Tensile recoil measurement of compressive strength for polymeric high performance fibers. J. Mater. Sci 1987, 22, 853–859. [Google Scholar]
- Roitman, DB; Wessling, RA; McAlister, J. Characterization of Poly(p-phenylene-cis-benzobisoxazole) in Methanesulfonic Acid. Macromolecules 1993, 26, 5174–5194. [Google Scholar]
- Roitman, DB; Tung, LH; Serrano, M. Polymerization Kinetics of Poly(P-phenylene-cis-benzobisoxazole). Macromolecules 1993, 26, 4045–4046. [Google Scholar]
- Serge, B; Xavier, F. Heat Resistance and Flammability of High Performance Fibers: A Review. Fire Mater 2002, 26, 155–168. [Google Scholar]
- Cohen, Y; Saruyama, Y; Thomas, EL. Crystal Solvates in the Poly [p-phenylene (benzo [1, 2-d: 4, 5-d'] bisthiazole-2, 6-diyl)]/poly (phosphoric acid)/water System. Macromolecules 1991, 24, 1161–1167. [Google Scholar]
- Tan, LS; Arnold, FE; Dang, TD; Chua, HH; Wei, KH. Pseudo-ladder Rigid-rod Polymers: Dihydroxy Pendent Benzothiazole Aromatic Heterocyclic Polymer and Copolymers. Polymer 1994, 35, 3091–3131. [Google Scholar]



| PBOH | PBO | |
|---|---|---|
| Reaction time/h | 15~18 | 24~26 |
| [η]/dL·g−1 | 18.1 | 21.6 |
| M̄w | 78000 | 84600 |
| PBOH | PBO | Zylon-AS | |
|---|---|---|---|
| Tensile strength / GPa | 3.1 ± 0.2 | 4.2 ± 0.2 | 5.8 ± 0.1 |
| tensile modulus / GPa | 155 ± 7 | 174 ± 5 | 190 ± 4 |
| Fibre Diameter /μ m | 18–20 | 18–20 | 11–13 |
| compressive strength / mPa | 331 ± 10 | 277 ± 9 | 297 ± 4 |
© 2008 by MDPI This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, H.; Huang, Y.-D.; Wang, F. Synthesis and Properties of Poly[p-(2,5-dihydroxy)- phenylenebenzobisoxazole] Fiber. Int. J. Mol. Sci. 2008, 9, 2159-2168. https://doi.org/10.3390/ijms9112159
Lin H, Huang Y-D, Wang F. Synthesis and Properties of Poly[p-(2,5-dihydroxy)- phenylenebenzobisoxazole] Fiber. International Journal of Molecular Sciences. 2008; 9(11):2159-2168. https://doi.org/10.3390/ijms9112159
Chicago/Turabian StyleLin, Hong, Yu-Dong Huang, and Feng Wang. 2008. "Synthesis and Properties of Poly[p-(2,5-dihydroxy)- phenylenebenzobisoxazole] Fiber" International Journal of Molecular Sciences 9, no. 11: 2159-2168. https://doi.org/10.3390/ijms9112159