Synthesis, Characterization, and Photovoltaic Properties of Soluble TiOPc Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1 Synthesis and characterization of TiOPc derivatives (3a and 3b)
2.2. Solubility of 3a and 3b
2.3. Optical properties of 3a and 3b
2.4. XRD
2.5. TEM studies
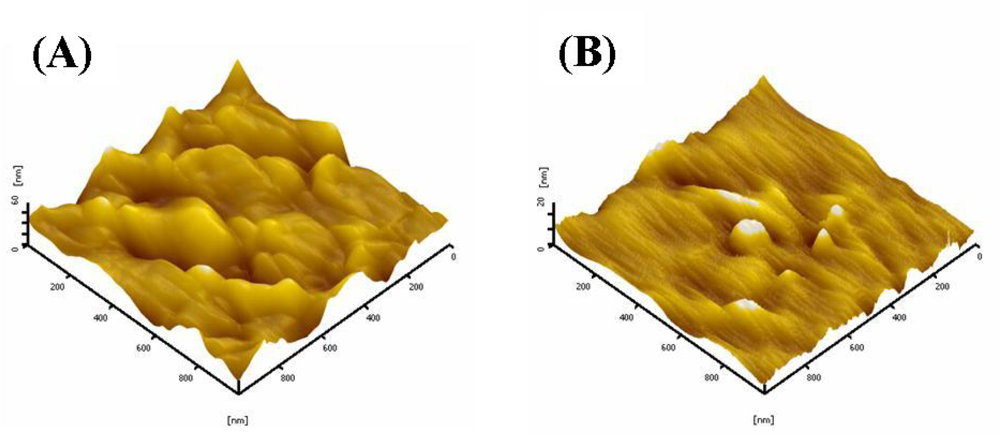
2.6. AFM studies
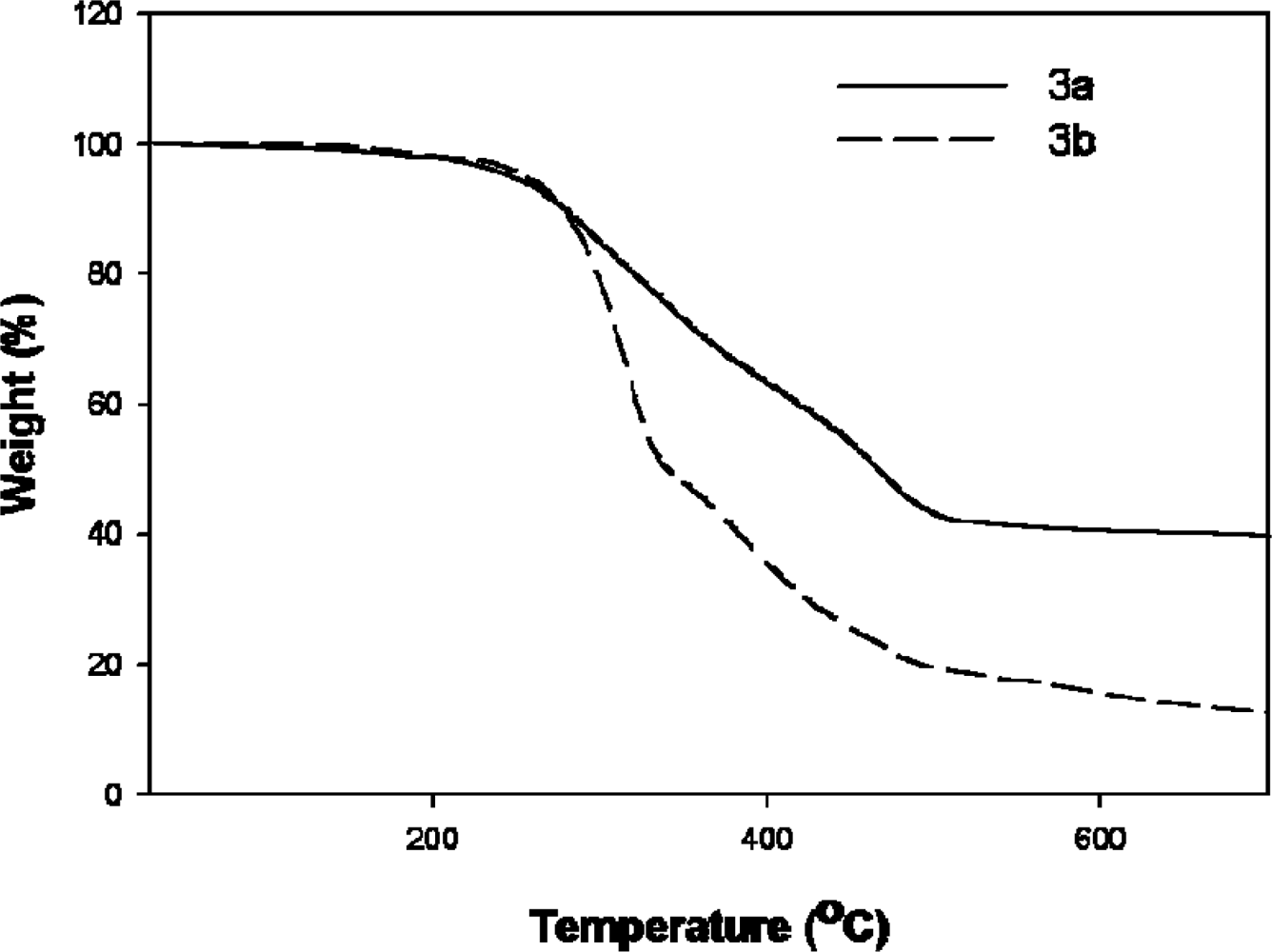
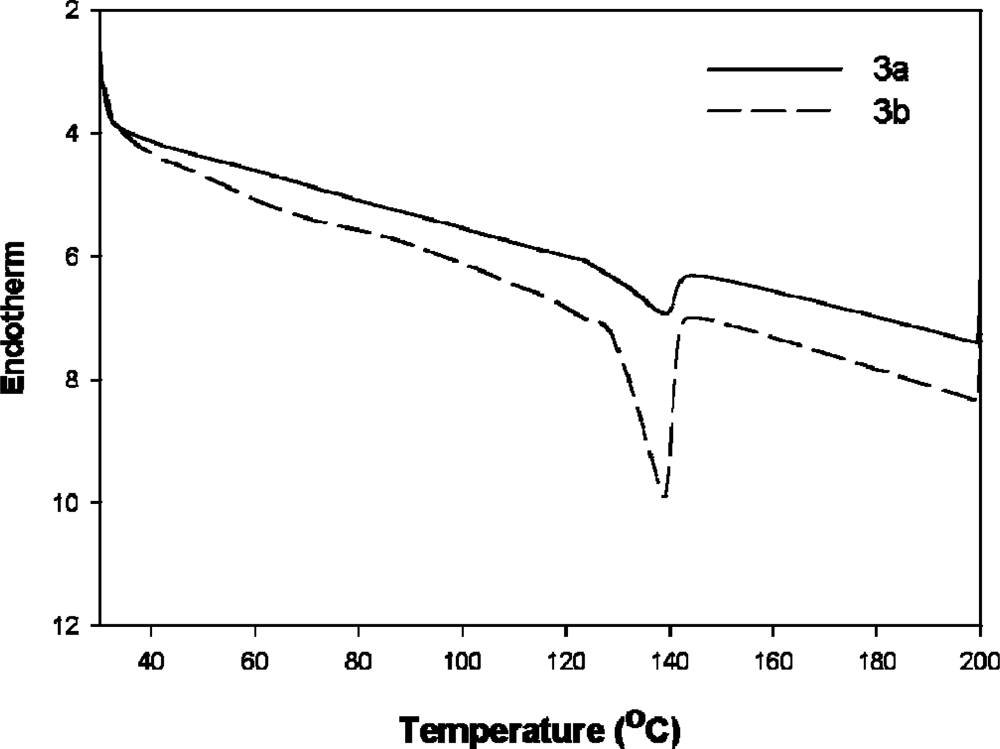
2.7. TGA and DSC studies
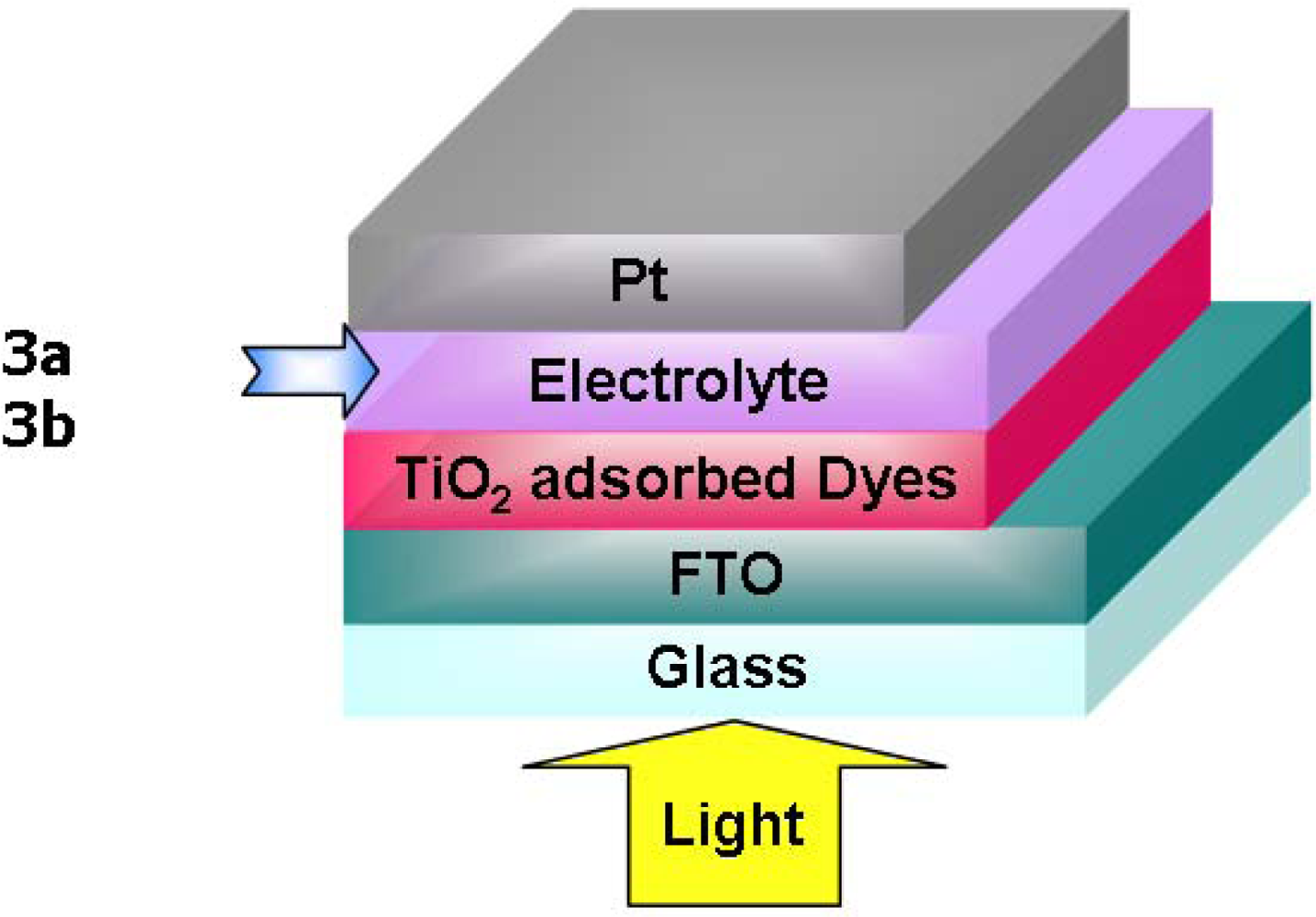
2.8. Photovoltaic performances of DSSC devices
3. Experimental Section
3.1. Materials
3.2. Measurements
3.3. Synthesis of TiOPc derivatives 3a and 3b
3.4. Fabrication of DSSC devices
4. Conclusions
Acknowledgments
References and Notes
- O’Regan, B; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar]
- Nazeeruddin, MK; Key, A; Rodico, I; Humphry-Baker, R; Muller, E; Liska, P; Vlachopoulos, N; Grätzel, M. Conversion of Light to Electricity by cis-X2Bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) Charge-Transfer Sensitizers (X = Cl−, Br−, I−, CN−, and SCN−) on Nanocrystalline TiO2 Electrodes. J. Am. Chem. Soc 1993, 115, 6382–6390. [Google Scholar]
- Smestad, G; Bignozzi, C; Argazzi, R. Testing of dye sensitized TiO2 solar cells I: Experimental photocurrent output and conversion efficiencies. Sol. Energy Mater. Sol. Cells 1994, 32, 259–272. [Google Scholar]
- Hara, K; Sayama, K; Ohga, Y; Shinpo, A; Suga, S; Arakawa, H. A coumarin-derivative dye sensitized nanocrystalline TiO2 solar cell having a high solar-energy conversion efficiency up to 5.6%. Chem. Commun 2001, 6, 569–570. [Google Scholar]
- Chen, J; Too, CO; Burrel, AK; Collis, GE; Officer, DL; Wallace, GG. Photovoltaic devices based on poly(bis-terthiophenes) and substituted poly(bisterthiophene). Synth. Met 2003, 137, 1373–1374. [Google Scholar]
- Li, G; Shrotriya, V; Yao, Y; Yang, Y. Investigation of annealing effects and film thickness dependence of polymer solar cells based on poly(3-hexylthiophene). J. Appl. Phys 2005, 98, 043704/1–043704/5. [Google Scholar]
- Tennakone, K; Kumara, GRRA; Kottegoda, IRM; Wijayantha, KGU; Perera, VPS. A solid-state photovoltaic cell sensitized with a ruthenium bipyridyl complex. J. Phys. D Appl. Phys 1998, 31, 1492–1496. [Google Scholar]
- O’Regan, B; Schwartz, DT; Zakeeruddin, SM; Grätzel, M. Electrodeposited nanocomposite n-p heterojunctions for solid-state dye-sensitized photovoltaics. Adv. Mater 2000, 12, 1263–1267. [Google Scholar]
- Woehrle, D; Eskes, M; Shigehara, K; Yamada, A. A simple synthesis of 4,5-disubstityted 1,2-dicyanobenzenes and 2,3,9,10,16,17,23,24-octasubstituted phthalocyanines. Synthesis 1993, 2, 194–196. [Google Scholar]
- Bach, U; Lupo, D; Comte, P; Moser, JE; Weissőrtel, F; Salbeck, J; Spreitzer, H; Grätzel, M. Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies. Nature 1998, 395, 583–585. [Google Scholar]
- Gebeyehu, D; Brabec, CJ; Sariciftci, NS; Vangeneugden, D; Kiebooms, R; Vanderzande, D; Kienberger, F; Schindler, H. Hybrid solar cells based on dye-sensitized nanoporous TiO2 electrodes and conjugated polymers as hole transport materials. Synth. Met 2002, 125, 279–287. [Google Scholar]
- Grant, CD; Schwartzberg, AM; Smestad, GP; Kowalik, J; Tolbert, LM; Zhang, JZ. Characterization of nanocrystalline and thin film TiO2 solar cells with poly(3-undecyl-2,2′-bithiophene) ans a sensitizer and hole conductor. J. Electroanal. Chem 2002, 522, 40–48. [Google Scholar]
- Bach, U; Tachibana, Y; Moser, JE; Haque, SA; Durrant, JR; Grätzel, M; Klug, DR. Charge Separation in Solid-State Dye-Sensitized Heterojunction Solar Cells. J. Am. Chem. Soc 1999, 121, 7445–7446. [Google Scholar]
- Li, W; Kang, J; Li, X; Fang, S; Lin, Y; Wang, G; Xiao, X. A novel polymer quaternary ammonium iodide and application in quasi-solid-state dye-sensitized solar cells. J. Photochem. Photobiol. A 2005, 170, 1–6. [Google Scholar]
- Matsumoto, H; Matsuda, T; Tsuda, T; Hagiwara, R; Ito, Y; Miyazaki, Y. The application on room temperature molten salt with low viscosity to the electrolyte for dye-sensitized solar cell. Chem. Letters 2001, 1, 26–27. [Google Scholar]
- Wang, H; Liu, X; Wang, Z; Li, H; Li, D; Meng, Q; Chen, L. Effect of Iodine Addition on Solid-State Electrolyte LiI/3-Hydroxypropionitrile (1:4) for Dye-Sensitized Solar Cells. J. Phys. Chem. B 2006, 110, 5970–5974. [Google Scholar]
- Xue, B; Wang, H; Hu, Y; Li, H; Wang, Z; Meng, Q; Huang, X; Sato, O; Chen, L; Fujishima, A. An alternative ionic liquid based electrolyte for dye-sensitized solar cells. Photochem. Photobiol. Sci 2004, 3, 918–919. [Google Scholar]
- Ng, DKP. Dendritic phthalocyanines: Synthesis, photophysical properties, and aggregation behavior. C.R. Chimie 2003, 6, 903–910. [Google Scholar]
- Gurek, AG; Bekaroglu, O. Tetrathia-macrocycle-bridged dimeric with hexakis(alkylthio) substituents and network polymer phthalocyanines. J. Porph. Phthalocyan 1997, 1, 227–237. [Google Scholar]
- Lee, HJ; Kim, WS; Park, SH; Shin, WS; Jin, SH; Lee, JK; Han, SM; Jung, KS; Kim, MR. Effect of nanocrystalline porous TiO2 films on interface adsorption of phthalocyanines and polymer electrolytes in dye-sensitized solar cells. Macromol. Symp 2006, 235, 230–236. [Google Scholar]
- Han, DS; Li, YJ; Kim, JS; Kim, E. Effect of bridging group in poly(titanyloxo-phthalocyanine)s on photocurrent generation. Synth. Met 1999, 101, 62–63. [Google Scholar]
- Han, DS; Lee, YJ; Kim, JS; Kim, E. Photocurrent generation of poly(titanyloxo-phthalocyanine)s and silica hybrid film. Synth. Met 2001, 117, 203–205. [Google Scholar]
- Yao, J; Yonehara, H; Pac, C. A Convenient Synthetic method for pure oxo(phthalo-cyaninato)titanium(IV) and application to other metal phthalocynines. Bull. Chem. Soc. Jpn 1995, 68, 1001–1005. [Google Scholar]
- Saito, T; Iwakabe, Y; Kobayashi, T; Suzuki, S; Iwayanagi, T. Thermochromism of specific crystal form oxotitanium phthalocyanines studied by electroabsorption and X-ray diffraction measurements. J. Phys. Chem 1994, 98, 2726–2728. [Google Scholar]




| Compounds | TiOPc | 3a | 3b |
|---|---|---|---|
| CHCl2 | I | S | S |
| Chlorobenzene | I | S | S |
| Toluene | I | S | S |
| Acetone | I | I | I |
| Methanol | I | I | I |
| DMF | I | I | I |
| THF | I | P (17 wt%) | P (26 wt%) |
| Compounds | XRD data 2 theta angle (dA) | Relative intensity (%) |
|---|---|---|
| 10.94 (8.08) | 73 | |
| 11.64 (7.59) | 79 | |
| 17.30 (5.12) | 25 | |
| 17.92 (4.94) | 31 | |
| 19.34 (4.58) | 84 | |
| 3a | 20.46 (4.34) | 37 |
| 22.50 (3.95) | 28 | |
| 22.84 (3.89) | 38 | |
| 23.50 (3.78) | 58 | |
| 24.38 (3.64) | 100 | |
| 26.60 (3.35)
| 38
| |
| 11.32 (7.81) | 83 | |
| 13.22 (6.69) | 41 | |
| 16.82 (5.27) | 38 | |
| 18.04 (4.91) | 33 | |
| 18.48 (4.80) | 96 | |
| 19.76 (4.49) | 63 | |
| 3b | 20.14 (4.41) | 77 |
| 21.28 (4.18) | 27 | |
| 22.06 (4.03) | 49 | |
| 23.00 (3.87) | 79 | |
| 23.64 (3.76) | 39 | |
| 24.86 (3.57) | 27 | |
| 25.98 (3.43) | 100 |
| Voc1) (V) | Jsc2)(mA/cm2) | FF3) | Efficiency (%) | |
|---|---|---|---|---|
| 3a | 0.61 | 8.49 | 0.53 | 2.73 |
| 3a with PEG4) | 0.65 | 9.84 | 0.54 | 3.49 |
| 3b | 0.61 | 10.02 | 0.53 | 3.19 |
| 3b with PEG4) | 0.66 | 10.04 | 0.54 | 3.62 |
| PEG | 0.67 | 8.98 | 0.49 | 2.94 |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/). This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, Y.-K.; Kang, H.-J.; Jang, Y.-W.; Lee, S.-B.; Lee, S.-M.; Jung, K.-S.; Lee, J.-K.; Kim, M.-R. Synthesis, Characterization, and Photovoltaic Properties of Soluble TiOPc Derivatives. Int. J. Mol. Sci. 2008, 9, 2745-2756. https://doi.org/10.3390/ijms9122745
Kim Y-K, Kang H-J, Jang Y-W, Lee S-B, Lee S-M, Jung K-S, Lee J-K, Kim M-R. Synthesis, Characterization, and Photovoltaic Properties of Soluble TiOPc Derivatives. International Journal of Molecular Sciences. 2008; 9(12):2745-2756. https://doi.org/10.3390/ijms9122745
Chicago/Turabian StyleKim, Young-Keun, Hyo-Jin Kang, Young-Wook Jang, Su-Bin Lee, Seung-Min Lee, Ki-Suck Jung, Jin-Kook Lee, and Mi-Ra Kim. 2008. "Synthesis, Characterization, and Photovoltaic Properties of Soluble TiOPc Derivatives" International Journal of Molecular Sciences 9, no. 12: 2745-2756. https://doi.org/10.3390/ijms9122745




