Synthesis and acidic properties of novel 3-methyl-4-[(2-amino-1,3,4-thiadiazol-5-il)-thioacetylamino]-4,5-dihydro-1H-1,2,4-triazol-5-one

Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References:
- Yüksek, H.; Ocak, Z.; Alkan, M.; Bahçeci, Ş.; Ozdemir, M. Molecules 2004, 9, 232–240.
- Yüksek, H.; Alkan, M.; Ocak, Z.; Bahçeci, Ş.; Ocak, M.; Ozdemir, M. Indian J. Chem. 2004, 43B, 1527–1531.
- Bahçeci, Ş.; Yüksek, H.; Ocak, Z.; Köksal, C.; Ozdemir, M. Acta Chim. Slov. 2002, 49, 783–794.
- Ikizler, A.A.; Un, R. Chim. Acta Turc. 1979, 7, 269–290, Chem. Abstr. 1981, 94, 15645d.
- Sample Availability: Available from the Authors.
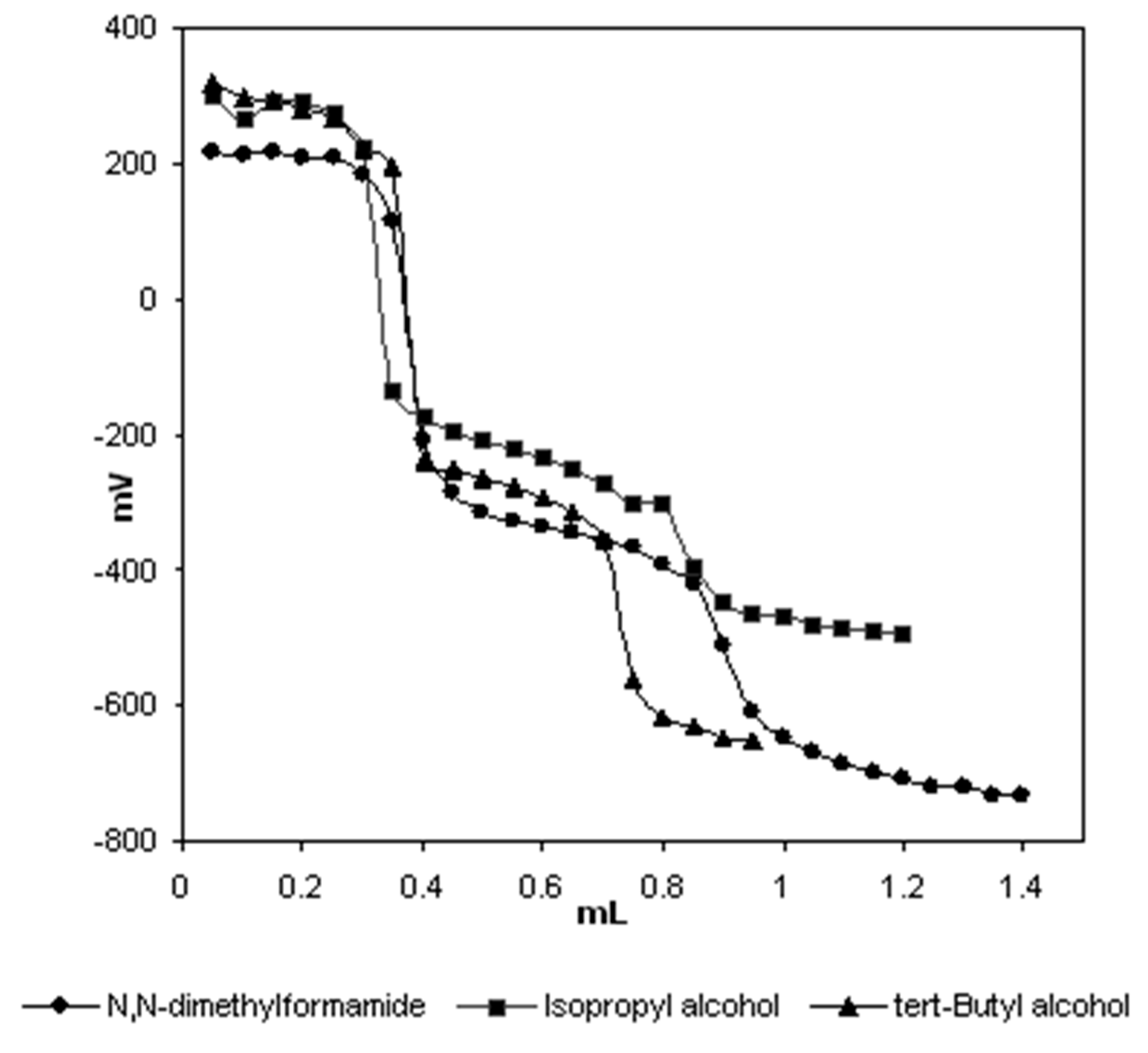
| Solvent | HNP1 (mV) | pKa1 | HNP2 (mV) | pKa2 |
|---|---|---|---|---|
| Isopropyl alcohol | 291 | 2.06 | -221 | 10.64 |
| tert-butyl alcohol | 287 | 2.16 | -269 | 11.61 |
| N,N-dimethylformamide | 218 | 3.36 | -342 | 12.68 |
© 2006 MDPI. All rights reserved.
Share and Cite
Yüksek, H.; Alkan, M.; Bahçeci, Ş. Synthesis and acidic properties of novel 3-methyl-4-[(2-amino-1,3,4-thiadiazol-5-il)-thioacetylamino]-4,5-dihydro-1H-1,2,4-triazol-5-one. Molbank 2006, 2006, M462. https://doi.org/10.3390/M462
Yüksek H, Alkan M, Bahçeci Ş. Synthesis and acidic properties of novel 3-methyl-4-[(2-amino-1,3,4-thiadiazol-5-il)-thioacetylamino]-4,5-dihydro-1H-1,2,4-triazol-5-one. Molbank. 2006; 2006(1):M462. https://doi.org/10.3390/M462
Chicago/Turabian StyleYüksek, Haydar, Muzaffer Alkan, and Şule Bahçeci. 2006. "Synthesis and acidic properties of novel 3-methyl-4-[(2-amino-1,3,4-thiadiazol-5-il)-thioacetylamino]-4,5-dihydro-1H-1,2,4-triazol-5-one" Molbank 2006, no. 1: M462. https://doi.org/10.3390/M462




