Synthesis of novel 2-(2-(6-chloro-9H-purin-9-yl)ethoxy)-6-isobutoxy-tetrahydro-2H-pyran-3-ol as a potential antiviral agent
Abstract
:Introduction
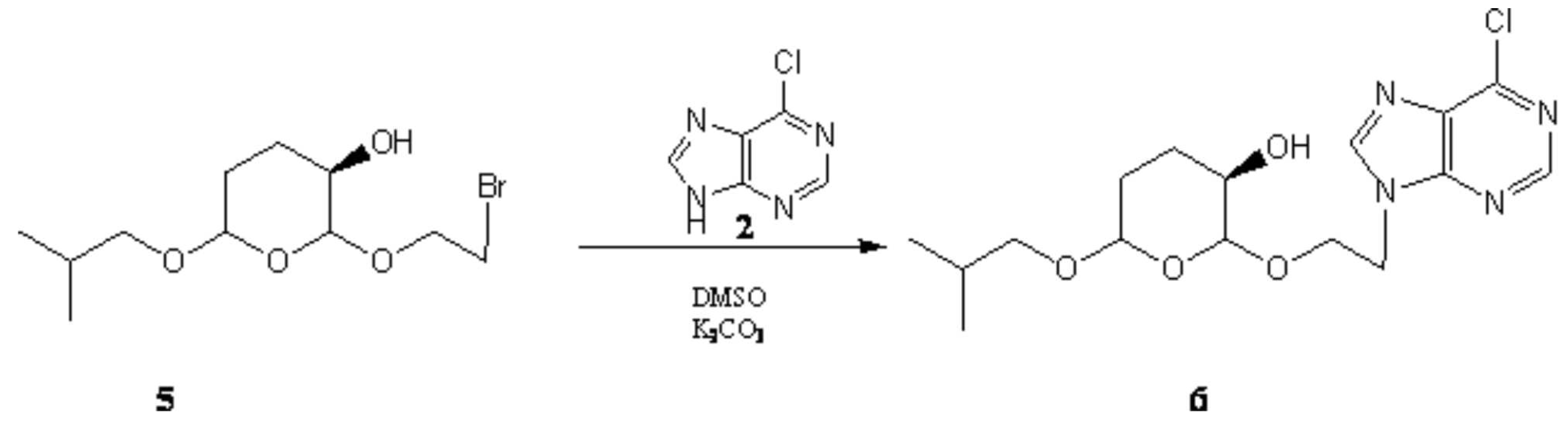
Results and Discussion
Experimental
General
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References
- Cheson, B. D. Hematol. Cell. Ther. (Suppl.) 1996, 38, 109.
- Bergmann, L. Leukemia (Suppl. 2) 1997, 11, 29.
- Borthwick, A. D.; Biggadike, K. Tetrahedron 1992, 48, 571.
- Agrofoglio, L.; Suhas, E.; Farese, A.; Condom, R.; Challand, S. R.; Earl, R. A.; Guedj, R. Tetrahedron 1994, 50, 10611.
- Huryn, D.; Okabe, M. Chem. Rev. 1992, 92, 1745.
- Mansour, T. S.; Storer, R. Curr. Pharm. Des. 1997, 3, 227.
- Ichikewa, E.; Kato, K. Curr. Med. Chem. 2001, 8, 385.
- Montgomery, J.; Hewson, K. J. J. Med. Chem. 1968, 11, 48.
- Parker, W. B.; King, S. A.; Allan, P. W.; Bennett, L. L.; Secrist, J. A.; Montgomery, J. A.; Gilbert, K. S.; Waud, W. R.; Wells, A. H.; Gillespie, G. Y.; Sorscher, E. J. Hum. Gene Ther. 1997, 8, 1637. [PubMed]
- Parker, W.B.; Allan, P. W.; Shaddix, S. C.; Rose, L. M.; Speegle, H. F.; Gillespie, G. Y.; Bennett, L. L. Biochem. Pharmacol. 1998, 55, 1673. [PubMed]
- Hocek, M.; Hol, A.; Votruba, I.; Dvorakova, H. J. Med. Chem. 2000, 43, 1817.
- Ogilvie, K. K.; Nguyen-BA, N. C. J. Chem. 1984, 62, 241.
- Sample Availability: Available from MDPI.

© 2006 MDPI. All rights reserved.
Share and Cite
Jarrahpour, A.A.; Torabi, E. Synthesis of novel 2-(2-(6-chloro-9H-purin-9-yl)ethoxy)-6-isobutoxy-tetrahydro-2H-pyran-3-ol as a potential antiviral agent. Molbank 2006, 2006, M481. https://doi.org/10.3390/M481
Jarrahpour AA, Torabi E. Synthesis of novel 2-(2-(6-chloro-9H-purin-9-yl)ethoxy)-6-isobutoxy-tetrahydro-2H-pyran-3-ol as a potential antiviral agent. Molbank. 2006; 2006(4):M481. https://doi.org/10.3390/M481
Chicago/Turabian StyleJarrahpour, A. A., and E. Torabi. 2006. "Synthesis of novel 2-(2-(6-chloro-9H-purin-9-yl)ethoxy)-6-isobutoxy-tetrahydro-2H-pyran-3-ol as a potential antiviral agent" Molbank 2006, no. 4: M481. https://doi.org/10.3390/M481



