Introduction
Our research group have reported that, under high dilution conditions, condensation reactions between 4,4’-ethylenebis(2,6-morpholinedione),
1, and aromatic diamines gave a new series of tetraaza chelating cylophanes, which have amide and aromatic groups in the ring framework and pendant carboxymethyl groups [
1]. The resulting functionalized macrocycles have novel coordination and structural properties due to the unique arrangement of different types of donor groups [
2]. In this work, we have employed the aromatic diamine 1,3-bis(aminomethyl)benzene,
2, and obtained a new chelating cyclophane (
3), 4,12,17,24-tetraoxo-6,9,19,22-tetrakis(carboxymethyl)-3,6,9,12,16,19,22,25-octaaza-
1,
4(1,3)-dibenzacyclohexacosano .
Spectroscopic Measurements
The solution electronic spectra were obtained by the use of a Perkin-Elmer Lambda 2 UV-vis spectrometer. The emission spectra were recorded on a Perkin Elmer LS-50 spectrofluorometer. The pH of the sample solutions was adjusted by adding a minimum amount of dilute NaOH solution or solid Na2CO3. The electrospray ionization (ESI) mass spectra were obtained by the use of a JEOL HX 110A spectrometer for sample solutions of an ammonia-methanol (5:95) mixture. The NMR spectra were obtained on a Bruker Avance 400 or Varian Unity 200 spectrometer in D2O with reference to sodium 2,2-dimethyl-2-silapentane-5-sulfonate, DSS. The infrared spectra were recorded on a Perkin-Elmer 1600 FT IR spectrophotometer and samples were analyzed as KBr pellets. The Elemental Analysis was performed by Centro de Investigaciones Químicas, Universidad Autónoma del Estado de Morelos. Cuernavaca, Mexico.
Anal. Calc. for C36H48N8O12(H2O)3: C,51.52; H,6.49; N, 13.36%; found: C,51.67; H,6.48; N, 13.47% .
1H NMR (D2O-Na2CO3, pD 10.3, 400 MHz, reference DSS): d = 7.29 (t, 2H, Hf), 7.15 (s, 2H, Hg), 7.13 (d, 4H, He), 4.24 (s, 8H, Hd), 3.17 (s, 8H, Hc), 3.09 (s, 8H, Hb),2.60 (s, 8H, Ha).
13C NMR (D2O-Na2CO3, pD 10.3, 50 MHz, reference DSS): d = 179.17 (-COO-), 174.14 (-CO-NH),138.30 (Ch), 129.32 (Cg), 128.90 (Cf), 126.76 (Ce), 59.18 (Cc), 58.43 (Cb), 52.59 (Ca),42.85 (Cd).
IR: 3335 cm-1 (N-H, amide), 1728 cm-1 (C=O, Carboxylate), 1668 cm-1 (-CO-NH-, Amide I, ), 1641 cm-1 (-CO-NH-, Amide II), 798 cm-1 (aromatic), 709 cm-1 (aromatic).
UV (aqueous solution, pH 9.0 I = 0.1 M KCl): 260 nm (e = 510 M-1 cm-1).
Fluorescence (aqueous solution, pH 9.0, I = 0.1 M KCl): Emission band at 290 nm (lexcitation = 260 nm).
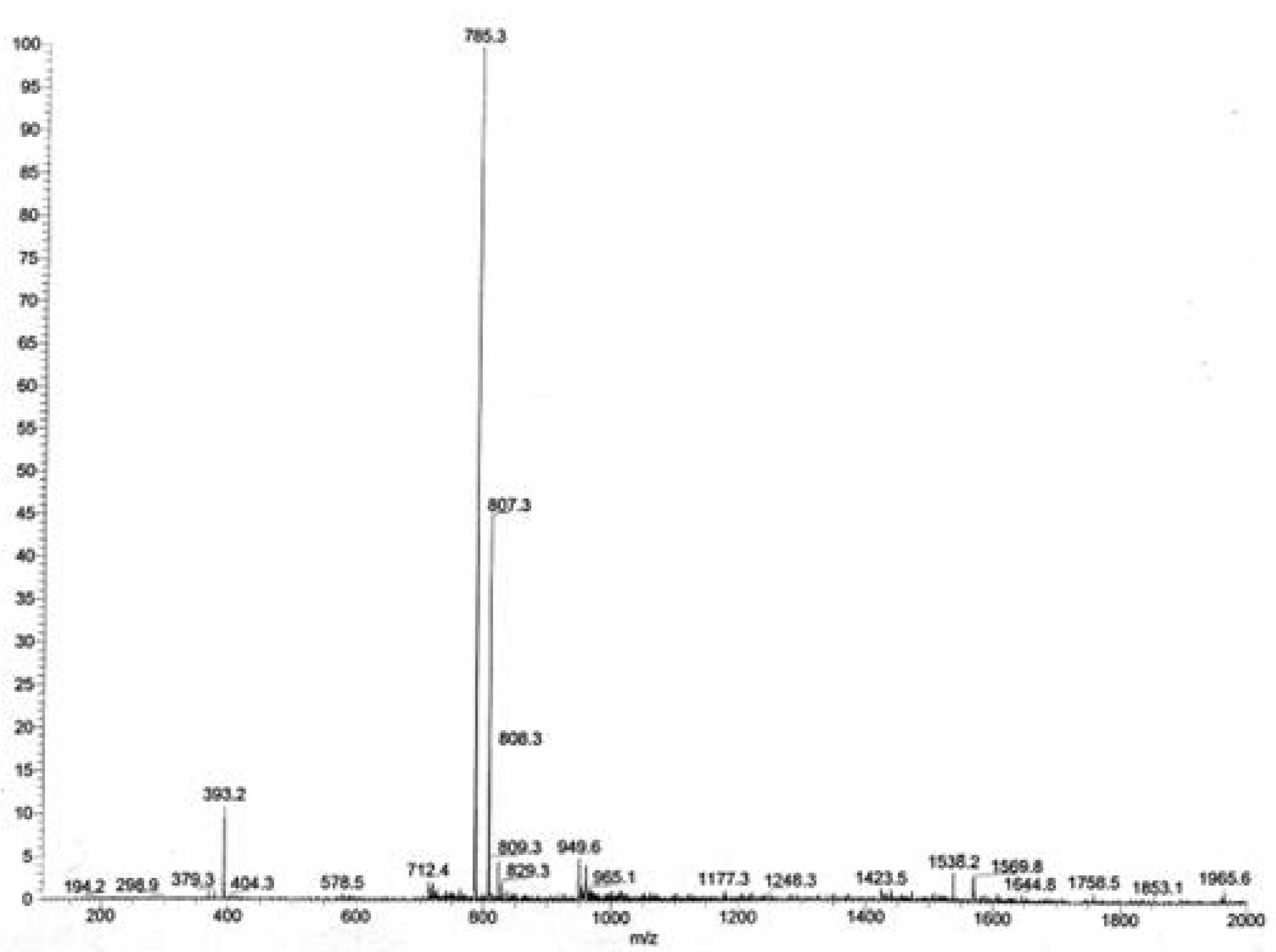
ESI MS: The electrospray mass spectrum of
3 in an ammoniacal methanol solution (
Figure 1) exhibited, in addition to the [M +H]
+ peak (
m/
z = 785.3, 100%), an extra peak at
m/
z = 393.2 (12%) [M+2H]
2+.The intervals between the isotope peaks proved that the latter species also had
z = 2.
Protonation Constants
Coordination [
2], molecular recognition [
3], spectroscopic[
1] and structural [
4] properties of this class of compounds are strongly pH dependent, therefore the precise determination of their acidity constants is very important. The protonation constants of the cyclophane were determinated by potentiometry. The titrations were carried out at 298.1 ± 0.1 K using KCl 0.1 M as supporting electrolyte in a sealed-jacketed vessel under nitrogen with a piston type burette and a Thermo Orion model 920Aplus pHmeter equipped with an Orion 8102U combination electrode. The glass electrode was calibrated as a hydrogen-ion concentration probe by titration of previously standardized amounts of HClO
4 with CO
2-free NaOH solutions and determinated the equivalent point by Gran’s method [
5], which gives the ionic product of water (pKw = -13.95). The computer program HYPERQUAD 2000 [
6] was used to calculate the protonation constants. The pH range investigated was 3 – 11 and the concentration of macrocycle was 1 x 10
–3 M.
Only 4 protonation constants, of the eight expected, were detected as log K
1 = 7.70 (standard deviation = 0.06), log K
2 = 6.63 (0.06), log K
3 = 3.91 (0.09) and log K
4 = 3.34 (0.10) and correspond to the stepwise basicity constants as described by the equilibrium equations:
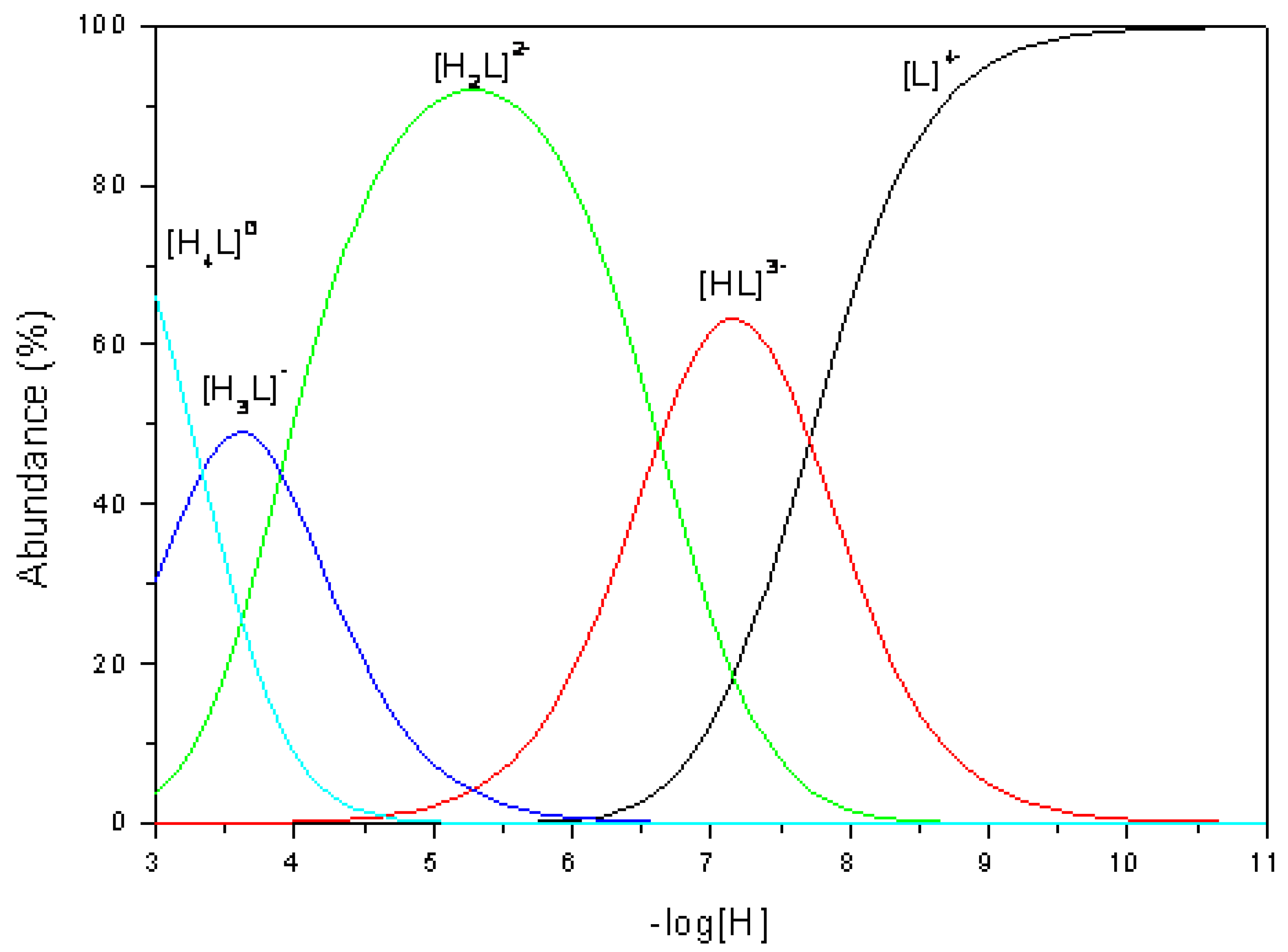
Distribution diagram of the various species present in solution was calculated using the program SPECIES (Academic Software) and is shown in
Figure 2.
Based on the reported data for other similar cyclophanes [
1,
2] is possible to assign the observed constants: first and second protonation events occur at amine nitrogen atoms, while third and fourth protonations occur at carboxylate oxygen atoms.