Synthesis and Physical Characterization of (E)-1-(3-morpholinopropyl)-3-phenoxy-4-styrylazetidine-2-one as the First β-lactam Bearing a Morpholino Moiety
Abstract
:Introduction
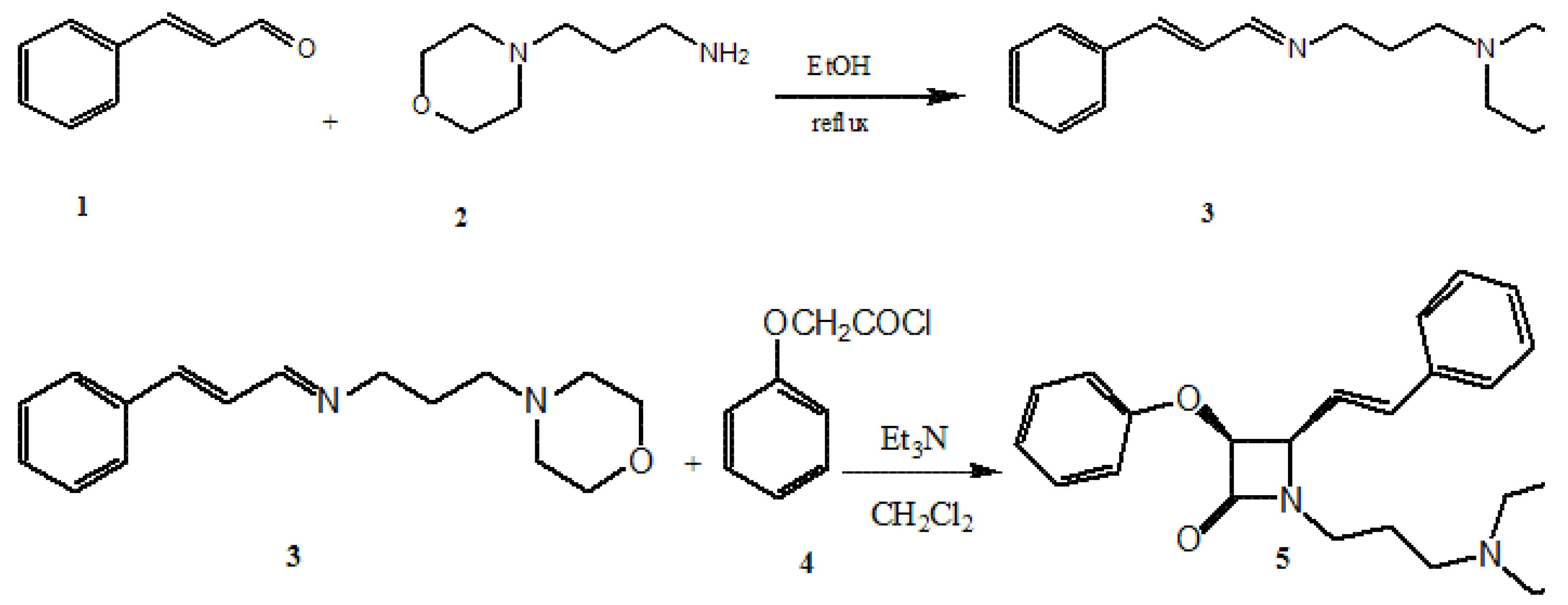
Results and Discussion
Experimental
General
Synthesis of 3-morpholino-N-((E)-3-phenylallylidene) propan-1-amine
Synthesis of 1-(3-morpholinopropyl)-3-phenoxy-4-styryl azetidine-2-one
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6References
- De Kimpe, N.; Abbaspour Tehrani, K.; Fonck, G. J. Org. Chem. 1996, 61, 6500–6503. [PubMed]
- Arrieta, A.; Cossio, F. P.; Lecea, B. J. Org. Chem. 2000, 65, 8458–8464. [PubMed]
- Besse, P.; Combourieu, B.; Poupin, P.; Sancelme, M.; Truffaut, N.; Veschambre, H.; De lort, A.M. J. Mol. Cat. B Enzymatic 1998, 5, 403–409.
- Frisch, M.J. GAUSSIAN03, Revision A.1; Frisch, M. J., et al., Eds.; Gaussian, Inc.: Pittsburgh PA, 2003. [Google Scholar]
- Foresman, J.B. Æ Frisch, Exploring Chemistry with Electronic Structure Methods, 2nd edition; Gaussian, INC: Pittsburgh, PA, 1996. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69, July 2001; National Institute of Standards and Technology: Gaithersburg, MD 20899.
- Jalbout, A.F.; Solimannejad, M.; Labonowski, J.K. Chem. Phys. Letts. 2003, 379, 503.
- Jalbout, A.F.; Jiang, Quasri, A.; Jeghnou, H.; Rhandour, A. Vib. Spect 2006, in press.
- Jalbout, A.F.; Nazara, F.; Turker, L. J. Mol. Struct. (THEOCHEM) 2004, 627, 1, (Invited Review).
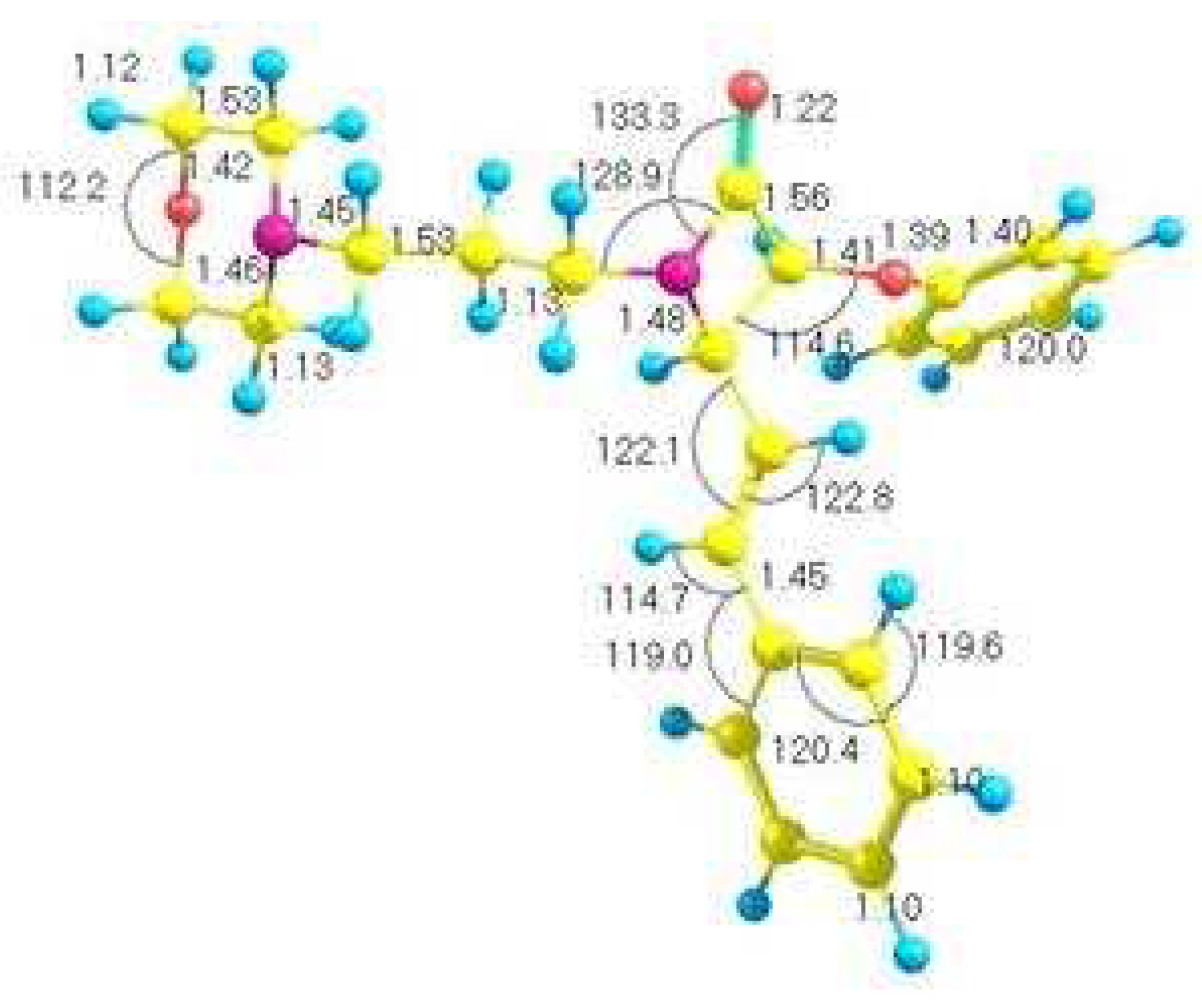
| Fitted Thermodynamic Equation (T/1000=t) | |
| Cp | -184.26382*t +2484.37019*t2 -1617.80676*t3+382.29145*t-2 |
| S | 96.58246*ln(t) + 2068.27*t -1006.92872*t2/2+109.15444 *t3/3 -0.34424/(2*t2) -11.32637 |
| ∆H | 403.63999*t +594.57262*t2/2-1.27691*t3/3+166.61048*t4/4 +7.63003/t -108.27816 |
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.
Share and Cite
Jarrahpour, A.; Jalbout, A.F.; Eskandari, M. Synthesis and Physical Characterization of (E)-1-(3-morpholinopropyl)-3-phenoxy-4-styrylazetidine-2-one as the First β-lactam Bearing a Morpholino Moiety. Molbank 2007, 2007, M542. https://doi.org/10.3390/M542
Jarrahpour A, Jalbout AF, Eskandari M. Synthesis and Physical Characterization of (E)-1-(3-morpholinopropyl)-3-phenoxy-4-styrylazetidine-2-one as the First β-lactam Bearing a Morpholino Moiety. Molbank. 2007; 2007(2):M542. https://doi.org/10.3390/M542
Chicago/Turabian StyleJarrahpour, Aliasghar, Abraham F. Jalbout, and Masomeh Eskandari. 2007. "Synthesis and Physical Characterization of (E)-1-(3-morpholinopropyl)-3-phenoxy-4-styrylazetidine-2-one as the First β-lactam Bearing a Morpholino Moiety" Molbank 2007, no. 2: M542. https://doi.org/10.3390/M542




