Further Aporphine Alkaloids from Phoebe lanceolata
Abstract
:1. Introduction
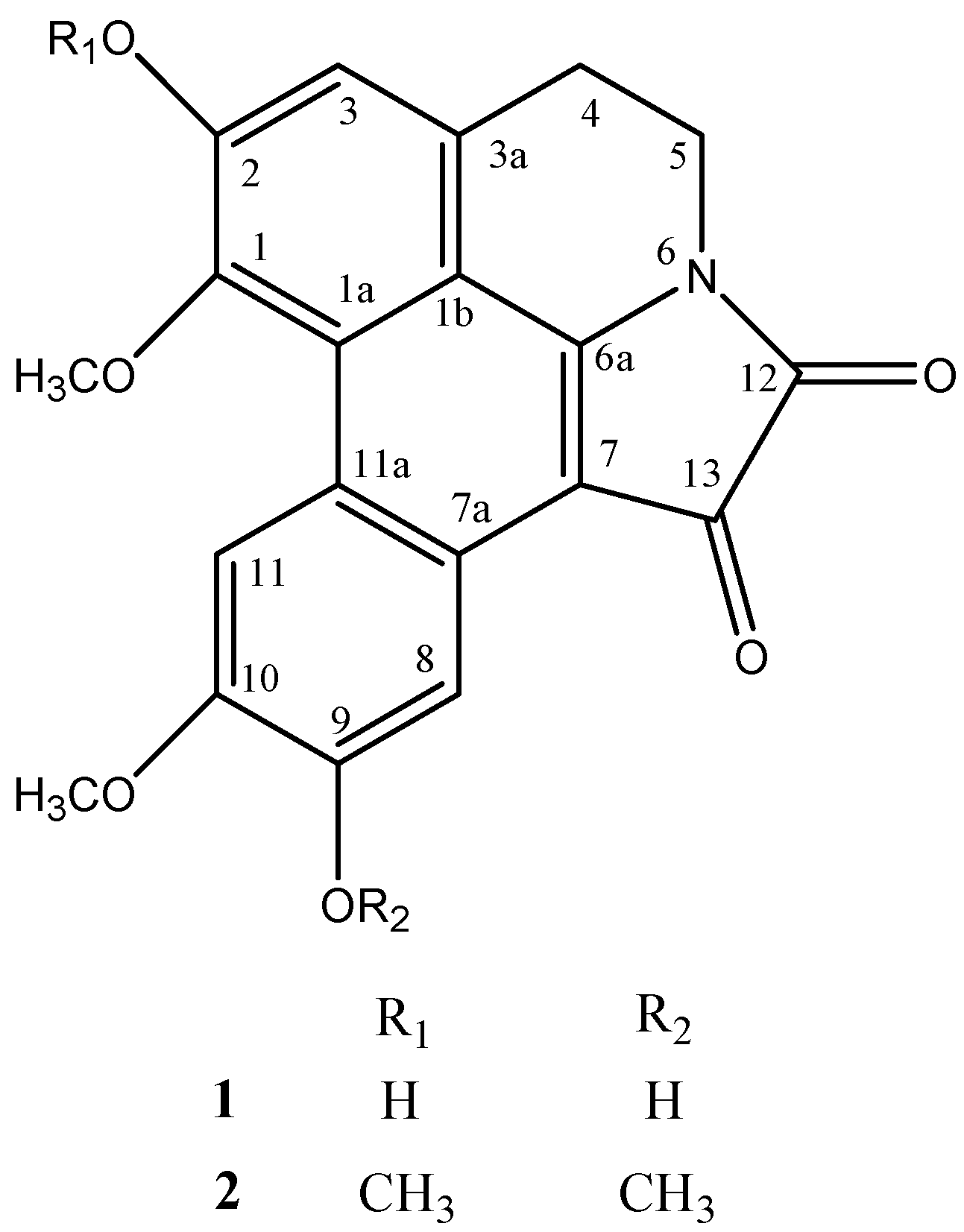
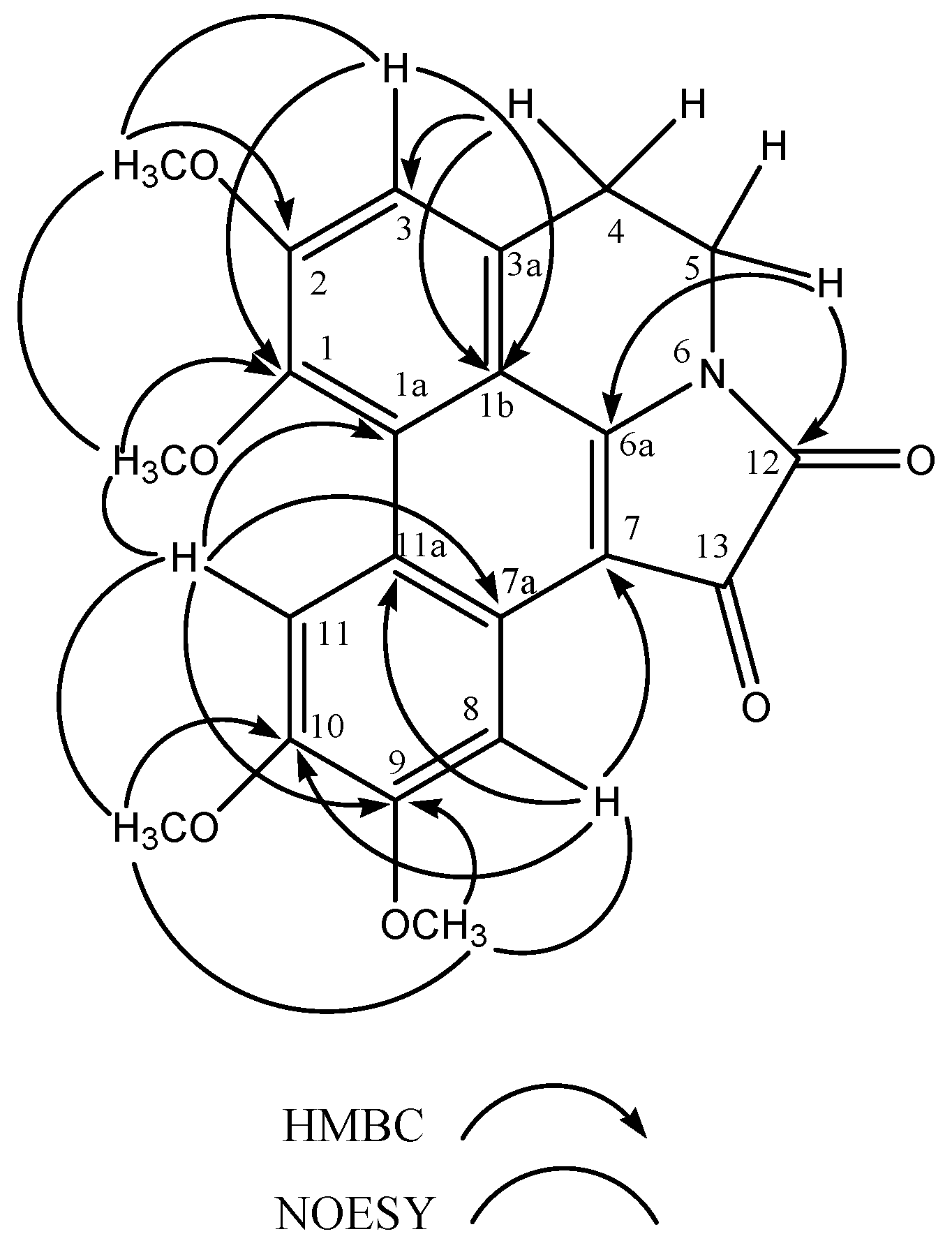
2. Results and discussion
3. Experimental
3.1. General
3.2. Plant material
3.3. Extraction and isolation
3.5. N-6/C-7 oxalyl-fused 1,2,9,10-tetramethoxy 6a,7-didehydroaporphine (2)
3.6. Methylation of compound 1
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6Acknowledgements
References
- Gaur, R.D. Flora of Garhwal North West Himalaya; Trans Media: Srinagar Garhwal, 1999; p. 59. [Google Scholar]
- Semwal, D.K.; Rawat, U. Nordelporphine alkaloid from bark of Phoebe lanceolata. Orient. J. Chem. 2007, 23, 771–772. [Google Scholar]
- Chen, C.C.; Huang, Y.L.; Lee, S.S.; Ou, J.C. A new oxalyl- fused aporphine alkaloid from Phoebe formosana. J. Nat. Prod. 1997, 60, 826–827. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Y.; Luo, D.; Li, B.; Zhang, G. Cytotoxic alkaloids from Boehmeria siamensis. Planta Med. 2003, 69, 842–845. [Google Scholar] [PubMed]
- Castedo, L.; Sal, C.; Sad, J.M.; Suau, R. Synthesis of oxoaporphines. An unusual photocyclization-photoreduction of 2,3-diaryl-Δ2-pyrroline-4,5-diones. J. Org. Chem. 1982, 47, 513–517. [Google Scholar] [CrossRef]
| Position | δC ppm | δH ppm ( J Hz) | DEPT | NOESY | HMBC |
|---|---|---|---|---|---|
| 1 | 144.54 | - | C | ||
| 1a | 126.20 | - | C | ||
| 1b | 127.95 | - | C | ||
| 2 | 159.28 | - | C | ||
| 3 | 114.56 | 7.12 s | CH | 3.63 | 127.95, 144.54 |
| 3a | 133.82 | - | C | ||
| 4 | 25.87 | 2.85 t (3.8) | CH2 | 3.18 | 114.56, 127.95 |
| 5 | 36.88 | 3.18 t (3.8) | CH2 | 2.85 | 151.47, 163.83 |
| 6a | 151.47 | - | C | ||
| 7 | 102.42 | - | C | ||
| 7a | 124.76 | - | C | ||
| 8 | 104.06 | 6.73 s | CH | 3.76 | 102.42, 115.83, 148.77 |
| 9 | 152.23 | - | C | ||
| 10 | 148.77 | - | C | ||
| 11 | 108.63 | 7.81 s | CH | 3.85, 3.92 | 124.76, 126.20, 152.23 |
| 11a | 115.83 | - | C | ||
| 12 | 163.83 | - | C | ||
| 13 | 173.33 | - | C | ||
| OCH3-1 | 58.85 | 3.92 s | CH3 | 3.63, 7.81 | 144.54 |
| OCH3-2 | 58.11 | 3.63 s | CH3 | 3.92, 7.12 | 159.28 |
| OCH3-9 | 56.36 | 3.76 s | CH3 | 3.85, 6.73 | 152.23 |
| OCH3-10 | 55.47 | 3.85 s | CH3 | 3.76, 7.81 | 148.77 |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Semwal, D.K.; Rawat, U.; Singh, G.J.P. Further Aporphine Alkaloids from Phoebe lanceolata. Molbank 2008, 2008, M581. https://doi.org/10.3390/M581
Semwal DK, Rawat U, Singh GJP. Further Aporphine Alkaloids from Phoebe lanceolata. Molbank. 2008; 2008(3):M581. https://doi.org/10.3390/M581
Chicago/Turabian StyleSemwal, Deepak K., Usha Rawat, and G. J. P. Singh. 2008. "Further Aporphine Alkaloids from Phoebe lanceolata" Molbank 2008, no. 3: M581. https://doi.org/10.3390/M581




