Preparation of 5-Bromo-2-naphthol: The Use of a Sulfonic Acid as a Protecting and Activating Group
Abstract
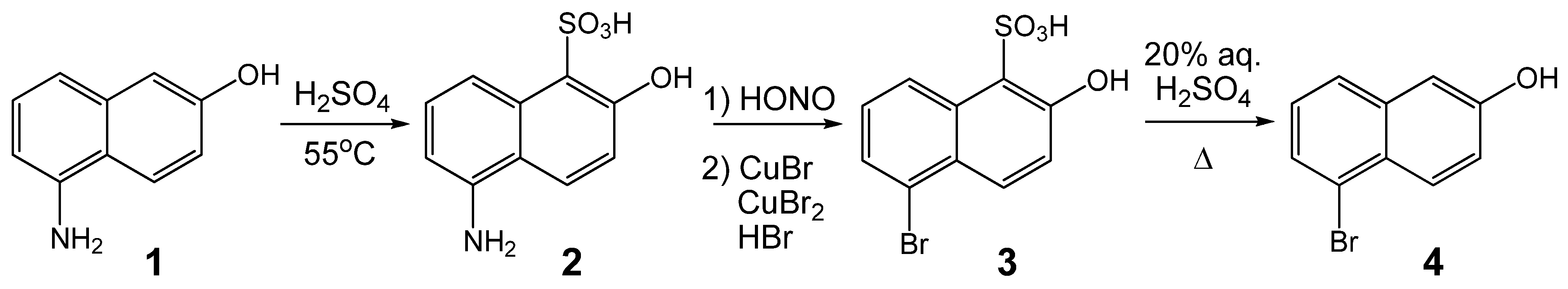
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General Methods
3.2. 5-Amino-2-hydroxynaphthalene-1-sulfonic acid
3.3. 5-Bromo-2-naphthol
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Goodman, R.H.; Fjeld, C.C.; Jackson, M.D. 1,1′-Binaphthyl-based inhibitors of NAD+-dependent deacetylase activity and SIR2-family members. PCT Int. Appl. WO 2007124383, 2007. [Google Scholar]
- Pearlman, M.B. Phenols from certain carboxylic acids. US 2764587, 1956. [Google Scholar]
- Dauben, W.G.; Saegebarth, K.A. Synthesis of β-(6-methoxy-1-naphthoyl)propionic acid. J. Am. Chem. Soc. 1951, 73, 1853–1854. [Google Scholar] [CrossRef]
- Bueno, A.B.; Flynn, C.J.; Gilmore, J.; Marcos, A.; Montero, C.; Porter, W.; Williams, A.C. A versatile protocol for the preparation of substituted 1- and 2-naphthyl piperazines from aminonaphthols. Tetrahedron Lett. 2005, 46, 7769–7771. [Google Scholar] [CrossRef]
- Ansink, H.R.W.; Zelvelder, E.; Cerfontain, H. Sulfonation of 1- and 2-Naphthol and their Methanesulfonate Esters with Sulfur Trioxide. The influence of initial sulfation on the sulfo-product composition. Recl. Trav. Chim. Pay. B. 1993, 112, 210–215. [Google Scholar] [CrossRef]
- Galli, C. Radical reactions of arenediazonium ions: An easy entry into the chemistry of the aryl radical. Chem. Rev. 1988, 88, 765–792. [Google Scholar] [CrossRef]
- Wahl, H.; Basilios, H. Preparation of 2-naphthoic acid and 2-halonaphthalenes. Bull. Soc. Chim. Fr. 1947, 482–484. [Google Scholar]
- Wolfe, W.C.; Doukas, H.M. 2-Bromonaphthalene. J. Chem. Ed. 1951, 28, 472–473. [Google Scholar] [CrossRef]
- Hanson, P.; Jones, J.R.; Taylor, A.B.; Walton, P.H.; Timms, A.W. Sandmeyer reactions. Part 7. An investigation into the reduction steps of Sandmeyer hydroxylation and chlorination reactions. J. Chem. Soc., Perkin Trans. 2 2002, 1135–1150. [Google Scholar] [CrossRef]
- Zollinger, H. Diazo Chemistry I: Aromatic and Heteroaromatic Compounds; VCH: Weinheim, 1994; pp. 210–265. [Google Scholar]
- Hanusek, J.; Macháček, V.; Lyčka, A. Reaction of 2-naphthol with substituted benzenediazonium salts in [bmim][BF4]. Dyes Pigments 2007, 73, 326–331. [Google Scholar], and ref therein.
- Fieser, L.F. 1,2-Aminonaphthol hydrochloride. Org. Synth. 1943, 2, 33–38. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Everett, R.; Hamilton, J.; Abelt, C. Preparation of 5-Bromo-2-naphthol: The Use of a Sulfonic Acid as a Protecting and Activating Group. Molbank 2009, 2009, M602. https://doi.org/10.3390/M602
Everett R, Hamilton J, Abelt C. Preparation of 5-Bromo-2-naphthol: The Use of a Sulfonic Acid as a Protecting and Activating Group. Molbank. 2009; 2009(3):M602. https://doi.org/10.3390/M602
Chicago/Turabian StyleEverett, Renata, Jillian Hamilton, and Christopher Abelt. 2009. "Preparation of 5-Bromo-2-naphthol: The Use of a Sulfonic Acid as a Protecting and Activating Group" Molbank 2009, no. 3: M602. https://doi.org/10.3390/M602



