Synthesis of 1-methyl-2-octynyl tetrahydro-2H-pyran-2-il ether (2)
To a stirred solution of 3-nonyn-2-ol (1) (200 mg, 1.43 mmol) in dry CH2Cl2 (8 mL) was added dihydropyran (0.20 mL, 2.15 mmol) and pyridinium p-toluenesulfonate (25 mg, 0.01 mmol) under an argon atmosphere at 0 °C. The reaction was allowed to continue at room temperature for 1 hour, after which time the mixture was poured into 20 mL of ice-water and extracted with CH2Cl2 (3 × 15 mL). The combined organic phases were washed with brine, dried (MgSO4), concentrated, and the crude purified by silica gel column chromatography yielding 2 (285 mg, 89% yield) as a yellowish oil: 1H NMR (300 MHz, CDCl3) δ/ppm = 0.87 (m, 3H), 1.29−1.31 (m, 6H), 1.41 (d, J = 7.4 Hz, 3H), 1.45−1.67 (m, 6H), 2.03 (m, 2H), 3.49−3.61 (m, 1H), 3.62−3.76 (m, 1H), 4.47−4.50 (m, 1H), 4.91 (br s, 1H); 13C NMR (75 MHz, CDCl3) δ/ppm = 13.9 (q), 18.5 (t), 19.5 (t), 22.0 (t), 22.2 (q), 25.4 (t), 28.3 (t), 30.6 (t), 33.9 (t), 61.1 (d), 62.2 (t), 81.3 (s), 85.2 (s), 98.8 (d); FT-IR (thin film) υmax (cm-1) 2875, 1458, 1310, 1115, 1091; FAB-MS m/z (relative intensity %) 224 [M]+ (11), 223 [M−1]+ (24), 153 [M−C5H11]+ (30), 85 (100). HMRS calculated for C14H24O2 [M]+ 224.177630, found 224.177120.
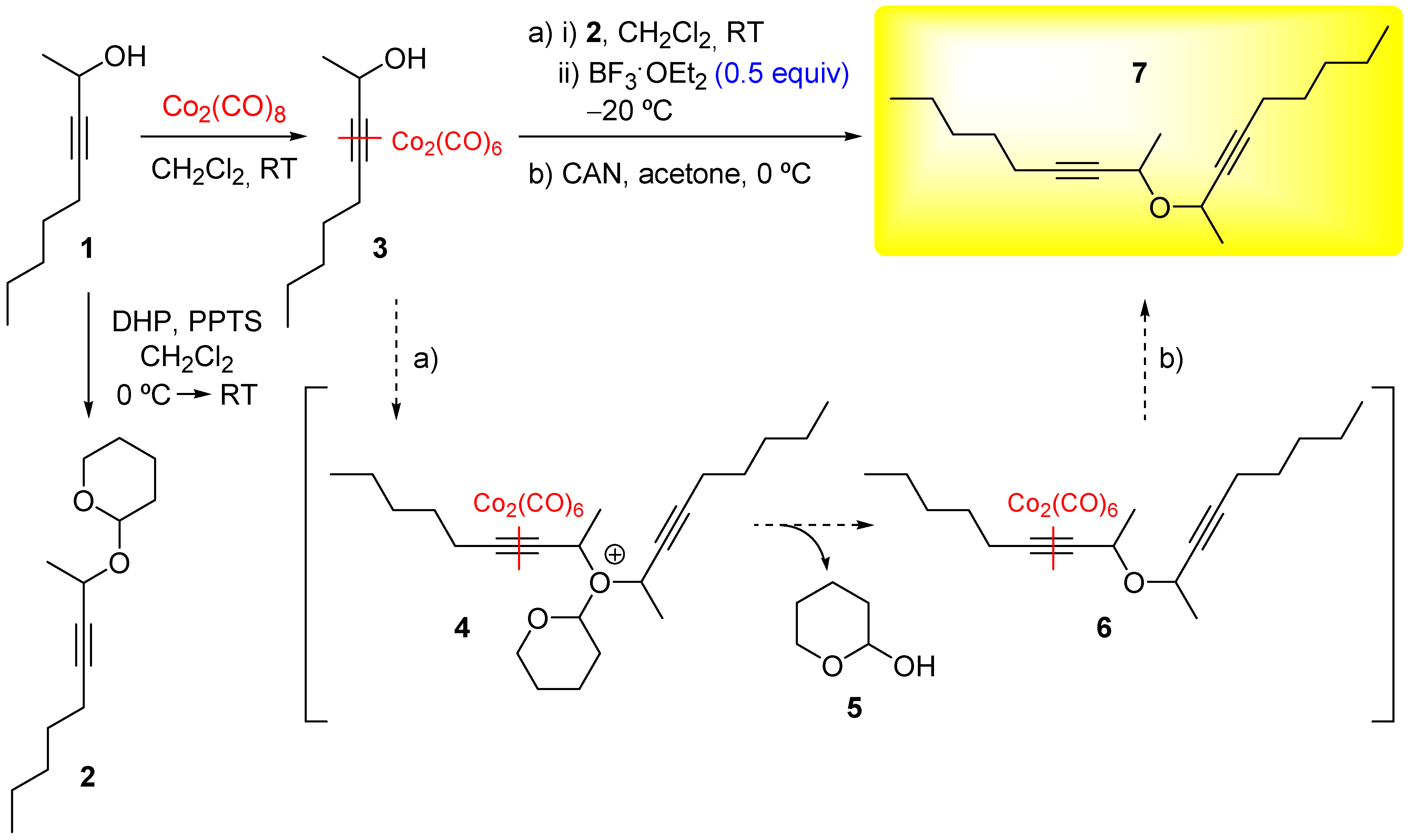
Synthesis of bis (1-methyl-2-octynyl) ether (7)
To a solution of alcohol 1 (100 mg, 0.71 mmol) was added dicobalt octadicarbonyl (297 mg, 0.86 mmol) in dry CH2Cl2 (7 mL) at room temperature. The reaction was stirred for 1 h, after which time the mixture was filtered through a pad of silica and the solvent evaporated to yield Co2(CO)6-propargylic ether 3 as a reddish oil. Complex 3 was dissolved in dry CH2Cl2 (7 mL) and THPO-protected propargylic alcohol 2 (302.4 mg, 1.35 mmol) was added. Then, BF3·OEt2 (29 μL, 0.23 mmol) was slowly added and the reaction mixture stirred for 1 h at −20 °C. The mixture was poured with vigorous stirring into a saturated solution of NaHCO3 (15 mL) and extracted with CH2Cl2 (2 × 15 mL). The combined organic phases were washed with brine, dried (MgSO4), and concentrated to obtain the crude Co2(CO)6-propargylic ether 6 as a reddish oil, which was used in the next step without further purification. The crude 6 was dissolved in acetone (5 mL) and the mixture cooled to 0 °C. Then, Ce(NO3)6(NH4)2 (480 mg, 0.88 mmol) was added in one portion and the mixture stirred for 5 min. The reaction mixture was concentrated and the resulting residue extracted with Et2O (3 × 10 mL). The combined organic phases were dried (MgSO4), concentrated, and the residue purified by silica gel column chromatography to yield 7 (83 mg, 45% overall yield) as a yellowish oil: 1H NMR (300 MHz, CDCl3) δ/ppm = 0.88 (t, J = 11.0 Hz, 6H), 1.27−1.37 (m, 6H), 1.40 (d, J = 6.5 Hz, 6H), 1.46−1.60 (m, 6H), 2.20 (ddd, J = 7.1, 7.1, 1.9 Hz, 4H), 4.43 (m, 2H)); 13C NMR (75 MHz, CDCl3) δ/ppm = 13.9 (q), 18.7 (t), 20.0 (q), 22.2 (t), 28.3 (t), 31.0 (t), 62.8 (d), 80.2 (s), 89.0 (s); FT-IR (thin film) υmax (cm-1) 2875, 1458, 1310, 1172, 1091; FAB-MS m/z (relative intensity %) 262 [M]+ (0.1), 247 [M−CH3]+ (34), 243 (14), 221 (17), 191 [M−C5H11]+ (5), 71 (100). Elemental analysis: Calculated for C18H30O: C, 82.38; H, 11.52. Found: C, 82.51; H, 11.80.