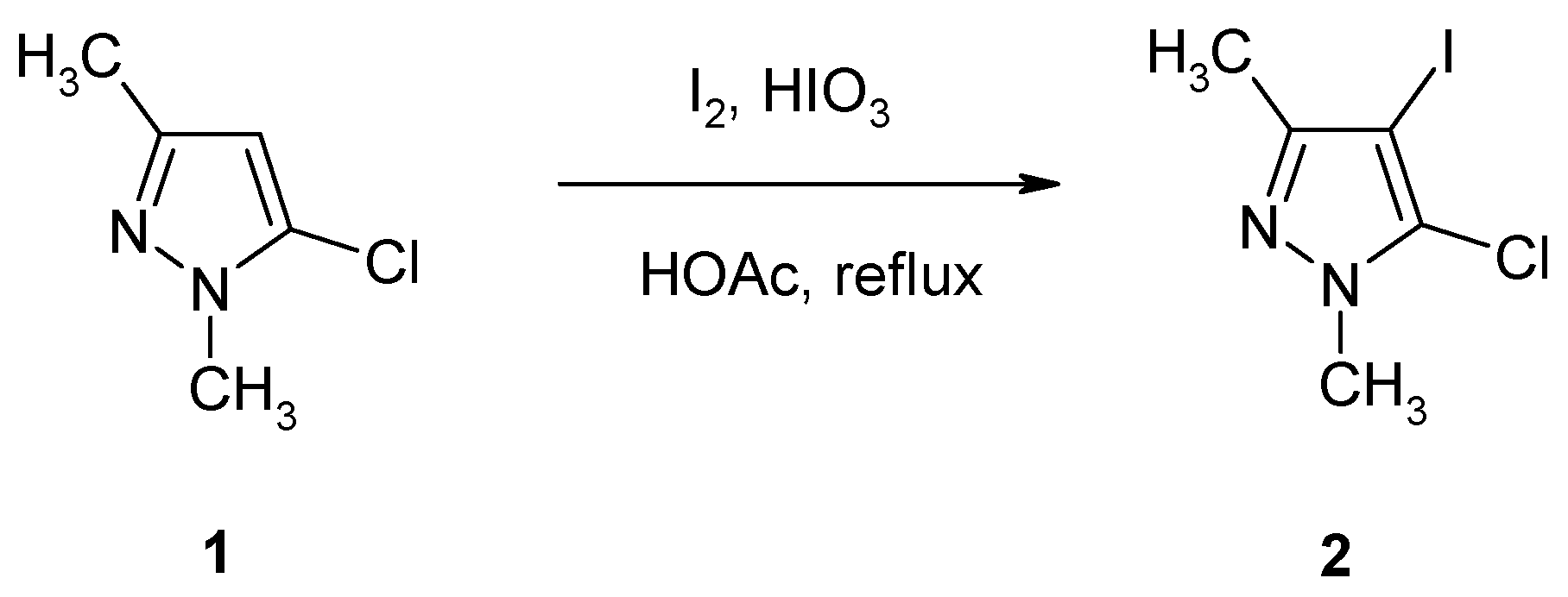
5-Chloro-4-iodo-1,3-dimethyl-1H-pyrazole
Abstract
:Experimental
5-Chloro-4-iodo-1,3-dimethyl-1H-pyrazole (2)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Negishi, E.; de Meijere, A. Handbook of Organopalladium Chemistry for Organic Synthesis; John Wiley & Sons: New York, NY, USA, 2002; Vol. 1 and Vol. 2. [Google Scholar]
- de Meijere, A.; Diederich, F. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: Weinheim, Germany, 2004; Vol. 1 and Vol. 2. [Google Scholar]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. The Sonogashira reaction: A booming methodology in synthetic organic chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Vasilevsky, S. F.; Tretyakov, E. V.; Elguero, J. Synthesis and properties of acetylenic derivatives of pyrazoles. Adv. Heterocycl. Chem. 2002, 82, 1–99. [Google Scholar]
- Heinisch, G.; Holzer, W.; Huber, T. Ein effizienter Zugang zu Aryl- oder Benzyl-4-pyrazolylketonen und –carbinolen. Arch. Pharm. (Weinheim) 1987, 320, 1267–1272. [Google Scholar] [CrossRef]
- Heinisch, G.; Holzer, W.; Obala, C. Beiträge zur Chemie von Pyrazolylalkinen. Monatsh. Chem. 1988, 119, 253–262. [Google Scholar] [CrossRef]
- Hahn, M.; Heinisch, G.; Holzer, W.; Schwarz, H. Synthesis of novel heteroaryl 4-pyrazolyl ketones. J. Heterocycl. Chem. 1991, 28, 1189–1192. [Google Scholar] [CrossRef]
- Arbačiauskienė, E.; Vilkauskaitė, G.; Eller, G. A.; Holzer, W.; Šačkus, A. Pd-catalyzed cross-coupling reactions of halogenated 1-phenylpyrazol-3-ols and related triflates. Tetrahedron 2009, 65, 7817–7824. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vilkauskaitė, G.; Eller, G.A.; Šačkus, A.; Holzer, W. 5-Chloro-4-iodo-1,3-dimethyl-1H-pyrazole. Molbank 2009, 2009, M620. https://doi.org/10.3390/M620
Vilkauskaitė G, Eller GA, Šačkus A, Holzer W. 5-Chloro-4-iodo-1,3-dimethyl-1H-pyrazole. Molbank. 2009; 2009(3):M620. https://doi.org/10.3390/M620
Chicago/Turabian StyleVilkauskaitė, Gytė, Gernot A. Eller, Algirdas Šačkus, and Wolfgang Holzer. 2009. "5-Chloro-4-iodo-1,3-dimethyl-1H-pyrazole" Molbank 2009, no. 3: M620. https://doi.org/10.3390/M620



