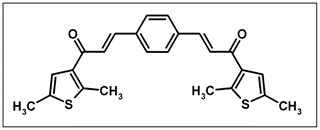
(2E,2'E)-3,3-(1,4-Phenylene)bis[1-(2,5-dimethyl-3-thienyl)prop- 2-en-1-one]
Abstract
:
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Dreoni, D.P.; Pinelli, D.; Trifiro, F. Synthesis of cyclohexanone oxime via ammoximation with molecular oxygen: The reaction network. J. Mol. Catal. 1991, 69, 171–190. [Google Scholar] [CrossRef]
- Yi, W.; Wu, X.; Cao, R.; Song, H.; Ma, L. Biological evaluations of novel vitamin C esters as mushroom tyrosinase inhibitors and antioxidants. Food Chem. 2009, 117, 381–386. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, P.; Zhang, X.; Zhang, Y.; Li, Y.; Wang, Y. Synthesis and biological evaluation of a novel series of 1,5-benzothiazepine derivatives as potential antimicrobial agents. Eur. J. Med. Chem. 2009, 44, 2815–2821. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Khan, R.A.; Ahmed, B. Syntheses and anti-depressant activity of 5-amino-1,3,4-thiadiazole-2-thiol imines and thiobenzyl derivative. Bioorg. Med. Chem. 2008, 16, 8029–8034. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.A.; Ahmed, E.A. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles. Eur. J. Med. Chem. 2009, 44, 2632–2635. [Google Scholar] [CrossRef] [PubMed]
- Tewtrakul, S.; Subhadhirasakul, S.; Karalai, C.; Ponglimanont, C.; Sarot Cheenpracha, S. Anti-inflammatory effects of compounds from Kaempferia parviflora and Boesenbergia pandurata. Food Chem. 2009, 115, 534–538. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asiri, A.M.; Khan, S.A. (2E,2'E)-3,3-(1,4-Phenylene)bis[1-(2,5-dimethyl-3-thienyl)prop- 2-en-1-one]. Molbank 2009, 2009, M636. https://doi.org/10.3390/M636
Asiri AM, Khan SA. (2E,2'E)-3,3-(1,4-Phenylene)bis[1-(2,5-dimethyl-3-thienyl)prop- 2-en-1-one]. Molbank. 2009; 2009(4):M636. https://doi.org/10.3390/M636
Chicago/Turabian StyleAsiri, Abdullah M., and Salman A. Khan. 2009. "(2E,2'E)-3,3-(1,4-Phenylene)bis[1-(2,5-dimethyl-3-thienyl)prop- 2-en-1-one]" Molbank 2009, no. 4: M636. https://doi.org/10.3390/M636