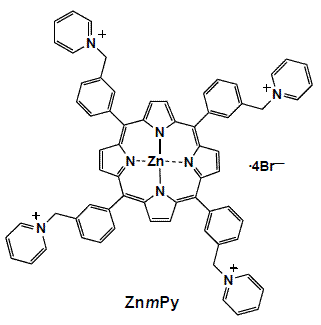
Zinc(II)-5,10,15,20-tetrakis(α-pyridino-m-tolyl)porphyrin Tetrabromide
Abstract
:Experimental
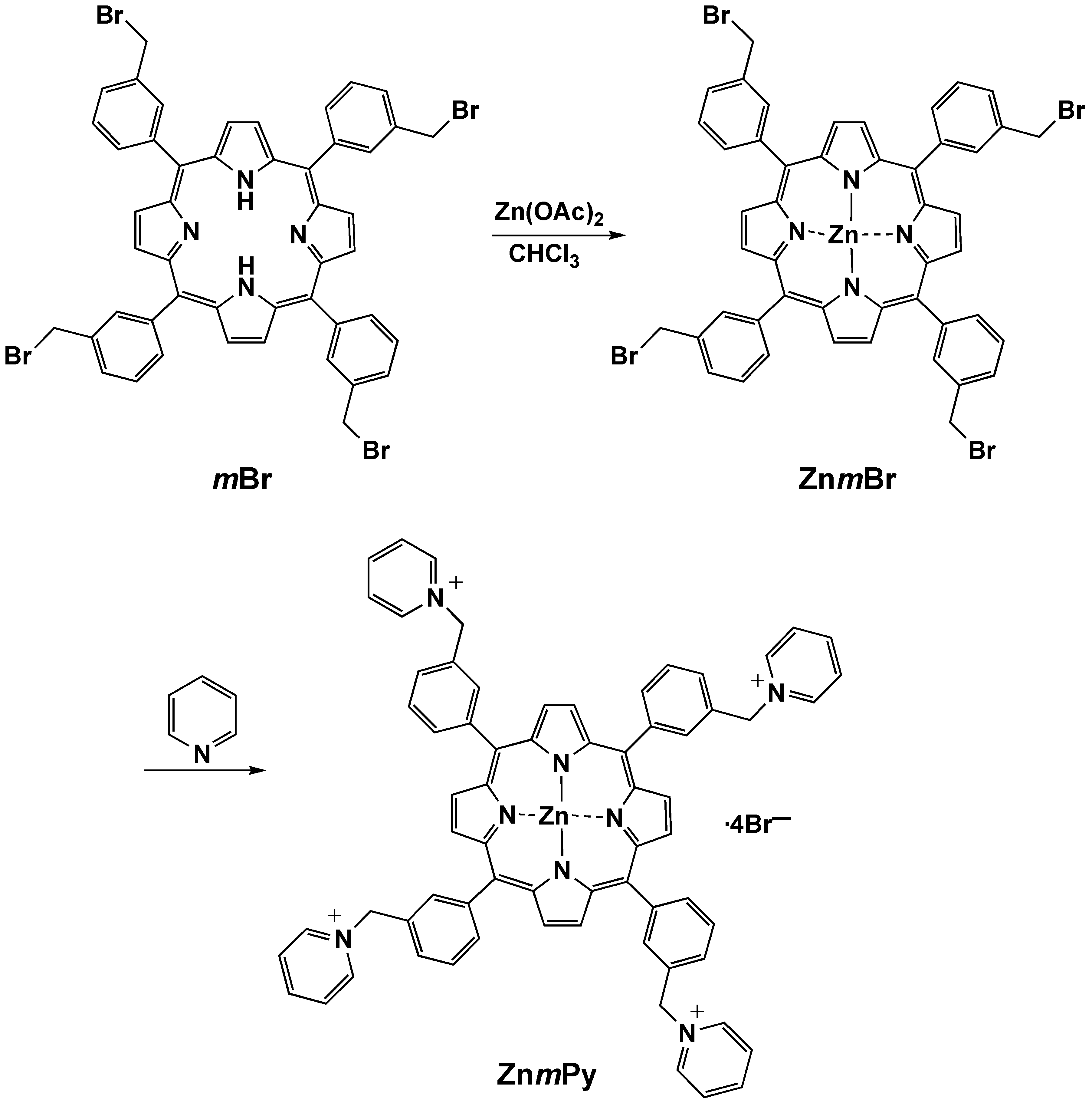
Zinc(II)-5,10,15,20-tetrakis(α-bromo-m-tolyl)porphyrin (ZnmBr)
Zinc(II)-5,10,15,20-tetrakis(α-pyridino-m-tolyl)porphyrin tetrabromide (ZnmPy)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Han, F.X.G.; Wheelhouse, R.T.; Hurley, L.H. Interactions of TMPyP4 and TMPyP2 with quadruplex DNA. Structural basis for the differential effects on telomerase inhibition. J. Am. Chem. Soc. 1999, 121, 3561–3570. [Google Scholar] [CrossRef]
- Izbicka, E.; Wheelhouse, R.T.; Raymond, E.; Davidson, K.K.; Lawrence, R.A.; Sun, D.Y.; Windle, B.E.; Hurley, L.H.; von Hoff, D.D. Effects of cationic porphyrins as G-quadruplex interactive agents in human tumor cells. Cancer Res. 1999, 59, 639–644. [Google Scholar] [PubMed]
- De Cian, A.; Cristofari, G.; Reichenbach, P.; de Lemos, E.; Monchaud, D.; Teulade-Fichou, M.P.; Shin-Ya, K.; Lacroix, L.; Lingner, J.; Mergny, J.L. Reevaluation of telomerase inhibition by quadruplex ligands and their mechanisms of action. Proc. Natl. Acad. Sci. USA 2007, 104, 17347–17352. [Google Scholar] [CrossRef] [PubMed]
- Mikami-Terao, Y.; Akiyama, M.; Yuza, Y.; Yanagisawa, T.; Yamada, O.; Kawano, T.; Agawa, M.; Ida, H.; Yamada, H. Antitumor activity of TMPyP4 interacting G-quadruplex in retinoblastoma cell lines. Exp. Eye. Res. 2009, 89, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Uno, T.; Ishikawa, Y. Stabilization of guanine quadruplex DNA by the binding of porphyrins with cationic side arms. Bioorg. Med. Chem. 2005, 13, 2423–2430. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Higashi, H.; Morioka, H. Molecular docking of porphyrins with cationic limbs on intramolecular G-quadruplex. Nucleic Acids Symp. Ser. 2007, 51, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, N.; Ishikawa, Y.; Uno, T. Solution properties and photonuclease activity of cationic bis-porphyrins linked with a series of aliphatic diamines. Chem. Pharm. Bull. 2001, 49, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Yamakawa, N.; Uno, T. Potent DNA photocleavage by zinc(II) complexes of cationic bis-porphyrins linked with aliphatic diamine. Bioorg. Med. Chem. 2002, 10, 1953–1960. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Yamakawa, N.; Uno, T. Synthetic control of interchromophoric interaction in cationic bis-porphyrins toward efficient DNA photocleavage and singlet oxygen production in aqueous solution. Bioorg. Med. Chem. 2007, 15, 5230–5238. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Yamakawa, N.; Uno, T. Binding of cationic bis-porphyrins linked with p- or m-xylylenediamine and their zinc(ii) complexes to duplex DNA. Molecules 2008, 12, 3117–3128. [Google Scholar] [CrossRef] [PubMed]
- Bookser, B.C.; Bruice, T.C. Syntheses of quadruply two- and three-atom, aza-bridged, cofacial bis(5,10,15,20-tetraphenylporphyrins). J. Am. Chem. Soc. 1991, 113, 4208–4218. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ishikawa, Y.; Yamashita, T.; Fujii, S.; Uno, T. Zinc(II)-5,10,15,20-tetrakis(α-pyridino-m-tolyl)porphyrin Tetrabromide. Molbank 2009, 2009, M637. https://doi.org/10.3390/M637
Ishikawa Y, Yamashita T, Fujii S, Uno T. Zinc(II)-5,10,15,20-tetrakis(α-pyridino-m-tolyl)porphyrin Tetrabromide. Molbank. 2009; 2009(4):M637. https://doi.org/10.3390/M637
Chicago/Turabian StyleIshikawa, Yoshinobu, Takeshi Yamashita, Satoshi Fujii, and Tadayuki Uno. 2009. "Zinc(II)-5,10,15,20-tetrakis(α-pyridino-m-tolyl)porphyrin Tetrabromide" Molbank 2009, no. 4: M637. https://doi.org/10.3390/M637