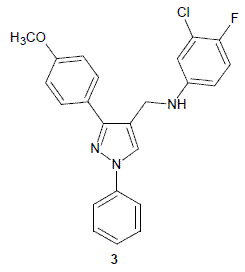
3-Chloro-4-fluoro-N-{[3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol- 4-yl]methyl}aniline
Abstract
:1. Introduction
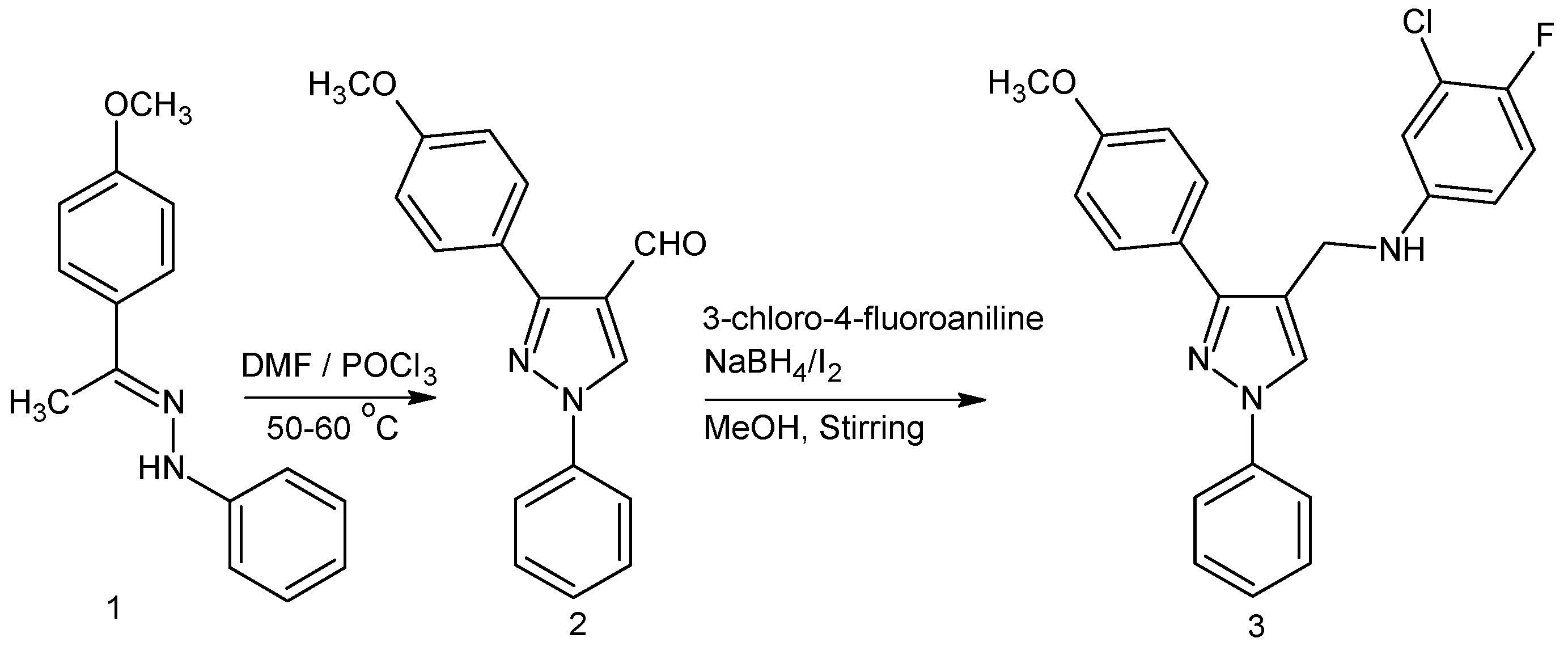
2. Result and Discussions
3. Experimental
2.1. 3-(4-Methoxyphenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde 2
2.2. 3-Chloro-4-fluoro-N-{[3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl]methyl}aniline 3
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Supplementary File 5Supplementary File 6Acknowledgement
References
- Alinezhad, H.; Tajbakhsh, M.; Salehian, F.; Fazil, K. Reductive amination of aldehydes and ketones to their corresponding amines with N-methylpyrrolidine zinc borohydride. Tetrahedron Lett. 2009, 50, 659–661. [Google Scholar] [CrossRef]
- Heydari, A.; Khaksar, S.; Akbari, J.; Esfandyari, M.; Pourayoubi, M.; Tajbakhsh, M. Direct reductive amination and selective 1,2-reduction of α,β-unsaturated aldehydes and ketones by NaBH4 using H3PW12O40 as catalyst. Tetrahedron Lett. 2007, 48, 1135–1138. [Google Scholar] [CrossRef]
- Saidi, M.R.; Brown, R.S.; Ziyaei-Halimjani, A. Reductive amination of aldehydes with sodium borohydride and lithium aluminum hydride in the presence of lithium perchlorate. J. Iran. Chem. Soc. 2007, 04, 194–198. [Google Scholar] [CrossRef]
- Baxter, E.W.; Reitz, A.B. Reductive aminations of carbonyl compounds with borohydride and borane reducing agents. In Organic Synthesis; Wiley InterScience: New York, NY, USA, 2002; Vol. 59. [Google Scholar]
- Bhandari, K.; Srivastava, S.; Shanker, G.; Nath, C. Substituted propanolamines and alkylamines derived from fluoxetine as potent appetite suppressants. Bioorg. Med. Chem. 2005, 13, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Delarue-Cochin, S.; Paunescu, E.; Maes, L.; Mouray, E.; Sergheraert, C.; Grellier, P.; Melnyk, P. Synthesis and antimalarial activity of new analogues of amodiaquine. Eur. J. Med. Chem. 2008, 43, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Varandas, L.S.; Fraga, C.A.M.; Miranda, A.L.P.; Barreiro, E.J. Design, synthesis and pharmacological evaluation of new nonsteroidal antiinflammatory 1,3,4-thiadiazole derivatives. Lett. Drug Des. Discovery 2005, 2, 62–67. [Google Scholar] [CrossRef]
- Kumar, S.; Bawa, S.; Drabu, S. N-[(2-Chloro-6-methylquinolin-3-yl)methyl]aniline. Molbank 2009, M618. [Google Scholar] [CrossRef]
- Prakash, O.; Pannu, K.; Kumar, A. Synthesis of some new 2-(3-aryl-1-phenyl-4-pyrazolyl)-benzoxazoles using hypervalent iodine mediated oxidative cyclization of Schiff bases. Molecules 2006, 11, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of the compound 3 is available from authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bawa, S.; Ahmad, F.; Kumar, S. 3-Chloro-4-fluoro-N-{[3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol- 4-yl]methyl}aniline. Molbank 2009, 2009, M640. https://doi.org/10.3390/M640
Bawa S, Ahmad F, Kumar S. 3-Chloro-4-fluoro-N-{[3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol- 4-yl]methyl}aniline. Molbank. 2009; 2009(4):M640. https://doi.org/10.3390/M640
Chicago/Turabian StyleBawa, Sandhya, Fasih Ahmad, and Suresh Kumar. 2009. "3-Chloro-4-fluoro-N-{[3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol- 4-yl]methyl}aniline" Molbank 2009, no. 4: M640. https://doi.org/10.3390/M640