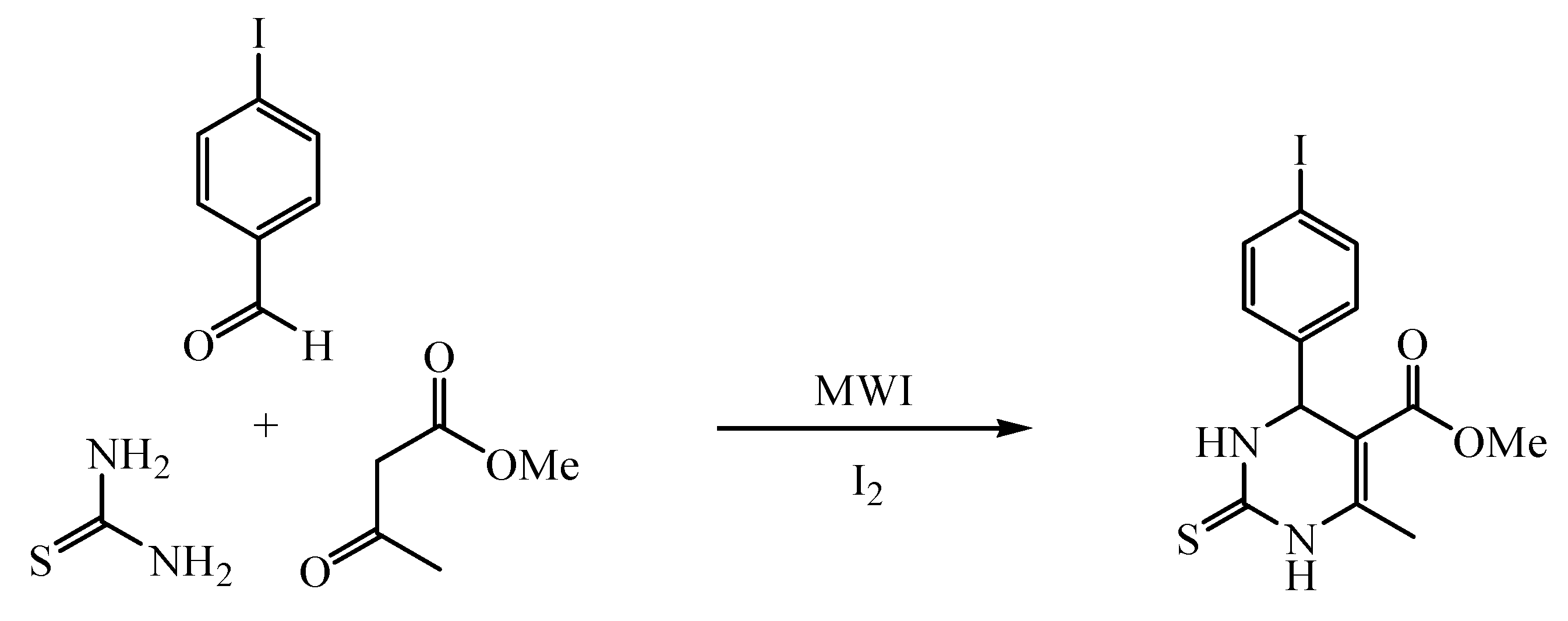
Methyl 6-Methyl-4-(4-iodophenyl)-1,2,3,4-tetrahydro-2-thioxo-5-pyrimidinecarboxylate
Abstract
:Experimental Procedure
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Kappe, C.O. 100 Years of Biginelli dihydropyrimidine synthesis. Tetrahedron 1993, 49, 6937–6963. [Google Scholar] [CrossRef]
- Kappe, C.O. Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog. Acc. Chem. Res. 2000, 33, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Biginelli, P. Aldehyde−urea derivatives of aceto-and oxaloacetic acids. Gazz. Chim. Ital. 1893, 23, 360–413. [Google Scholar]
- Sanjeev, P.; Gokavi, G.S. Heteropoly acid catalyzed synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Catal. Commun. 2007, 8, 279–284. [Google Scholar]
- Walsh, P.J.; Li, H.M.; de Parrodi, C.A. A green chemistry approach to asymmetric catalysis: solvent-free and highly concentrated reactions. Chem. Rev. 2007, 107, 2503–2545. [Google Scholar] [CrossRef] [PubMed]
- Saxena, I.; Borah, D.C.; Sarma, J.C. Three component condensations catalyzed by iodine–alumina for the synthesis of substituted 3,4-dihydropyrimidin-2(1H)-ones under microwave irradiation and solvent-free condition. Tetrahedron Lett. 2005, 46, 1159–1160. [Google Scholar] [CrossRef]
- Bhosale, R.S.; Bhosale, S.V.; Bhosale, S.V.; Wang, T.; Zubaidha, P.K. An efficient, high yield protocol for the one-pot synthesis of dihydropyrimidin-2(1H)-ones catalyzed by iodine. Tetrahedron Lett. 2004, 45, 9111–9113. [Google Scholar] [CrossRef]
- Ko, S.; Sastry, M.N.V.; Lin, C.; Yao, C.-F. Molecular iodine-catalyzed one-pot synthesis of 4-substituted-1,4-dihydropyridine derivatives via Hantzsch reaction. Tetrahedron Lett. 2005, 46, 5771–5774. [Google Scholar] [CrossRef]
- Srinivas, K.V.N.S.; Das, B. Iodine-catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones and thiones: A simple and efficient procedure for the Biginelli reaction. Synthesis 2004, 13, 2091–2093. [Google Scholar]
- de la Hoz, A.; Diaz-Ortiz, A.; Moreno, A.; Langa, F. Cycloadditions under microwave irradiation conditions: Methods and applications. Eur. J. Org. Chem. 2000, 3659–3673. [Google Scholar] [CrossRef]
- Varma, R.S. Solvent-free synthesis of heterocyclic compounds using microwaves. J. Heterocycl. Chem. 1999, 36, 1565–1571. [Google Scholar] [CrossRef]
- Varma, R.S. Solvent-free organic syntheses using supported reagents and microwave irradiation. Green Chem. 1999, 1, 43–55. [Google Scholar] [CrossRef]
- Loupy, A.; Petit, A.; Hamelin, J.; Texier-Boullet, F.; Jacquault, P.; Mathe, D. New solvent-free organic synthesis using focused microwaves. Synthesis 1998, 1213–1234. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pan, N.; Zhang, W.; Liu, Q. Methyl 6-Methyl-4-(4-iodophenyl)-1,2,3,4-tetrahydro-2-thioxo-5-pyrimidinecarboxylate. Molbank 2009, 2009, M645. https://doi.org/10.3390/M645
Pan N, Zhang W, Liu Q. Methyl 6-Methyl-4-(4-iodophenyl)-1,2,3,4-tetrahydro-2-thioxo-5-pyrimidinecarboxylate. Molbank. 2009; 2009(4):M645. https://doi.org/10.3390/M645
Chicago/Turabian StylePan, Ning, Wenwen Zhang, and Qingjian Liu. 2009. "Methyl 6-Methyl-4-(4-iodophenyl)-1,2,3,4-tetrahydro-2-thioxo-5-pyrimidinecarboxylate" Molbank 2009, no. 4: M645. https://doi.org/10.3390/M645



