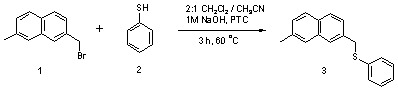
2-Methyl-7-(phenylsulfanylmethyl)naphthalene
Abstract
:1. Introduction
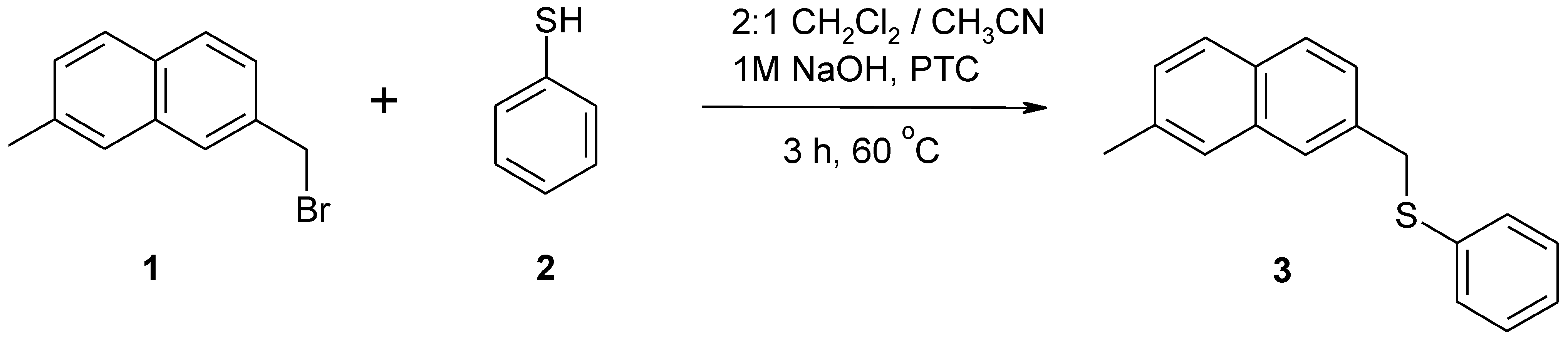
2. Experimental
2.1. General
2.2. 2-Methyl-7-(phenylsulfanylmethyl)naphthalene (3)
3. Conclusion
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Maslak, P.; Narvaez, J.N. Mesolytic ceavage of C-C bonds. Comparison with homolytic and heterolytic processes in the same substrate. Angew. Chem. Int. Ed. Engl. 1990, 29, 283–285. [Google Scholar] [CrossRef]
- Frankel, M.; Gertner, D.; Jacobson, H.; Zilkha, A. Syntheses of Poly-S-Alkyl-L-Cysteines. J. Chem. Soc. 1960, 1390–1393. [Google Scholar] [CrossRef]
- Dymicky, M.; Byler, D.M. Synthesis of ethyl n-carbobenzoxytyrosyl-s-benzylcysteinylglycinate. Org. Prep. Proced. Int. 1991, 23, 93–101. [Google Scholar] [CrossRef]
- Vögtle, F.; Klieser, B. Cesium-ion assisted synthesis of strained compounds. Synthesis 1982, 294–296. [Google Scholar] [CrossRef]
- Yin, J.M.; Pidgeon, C. A simple and efficient method for preparation of unsymmetrical sulfides. Tetrahedron Lett. 1997, 38, 5953–5954. [Google Scholar] [CrossRef]
- Herriott, A.W.; Picker, D. Phase-transfer synthesis of sulfides and dithioacetals. Synthesis 1975, 447–448. [Google Scholar] [CrossRef]
- Maslak, P.; Guthrie, R.D. Electron apportionment in cleavage of radical-anions. 2. naphthylmethyl phenyl ethers vs. naphthyl benzyl ethers. J. Am. Chem. Soc. 1986, 108, 2637–2640. [Google Scholar] [CrossRef]
- Buuhoi, N.P.; Lecocq, J. Side-chain bromination of some alkylnaphthalenes with n-bromosuccinimide. J. Chem. Soc. 1946, 830–832. [Google Scholar] [CrossRef]
- McMurry, J. Organic Chemistry, 5th ed.; Brooks/Cole: Pacific Grove, CA, USA, 2000; p. 729. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bogdanov, J.; Maslak, P. 2-Methyl-7-(phenylsulfanylmethyl)naphthalene. Molbank 2010, 2010, M670. https://doi.org/10.3390/M670
Bogdanov J, Maslak P. 2-Methyl-7-(phenylsulfanylmethyl)naphthalene. Molbank. 2010; 2010(2):M670. https://doi.org/10.3390/M670
Chicago/Turabian StyleBogdanov, Jane, and Przemyslaw Maslak. 2010. "2-Methyl-7-(phenylsulfanylmethyl)naphthalene" Molbank 2010, no. 2: M670. https://doi.org/10.3390/M670