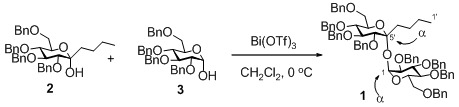
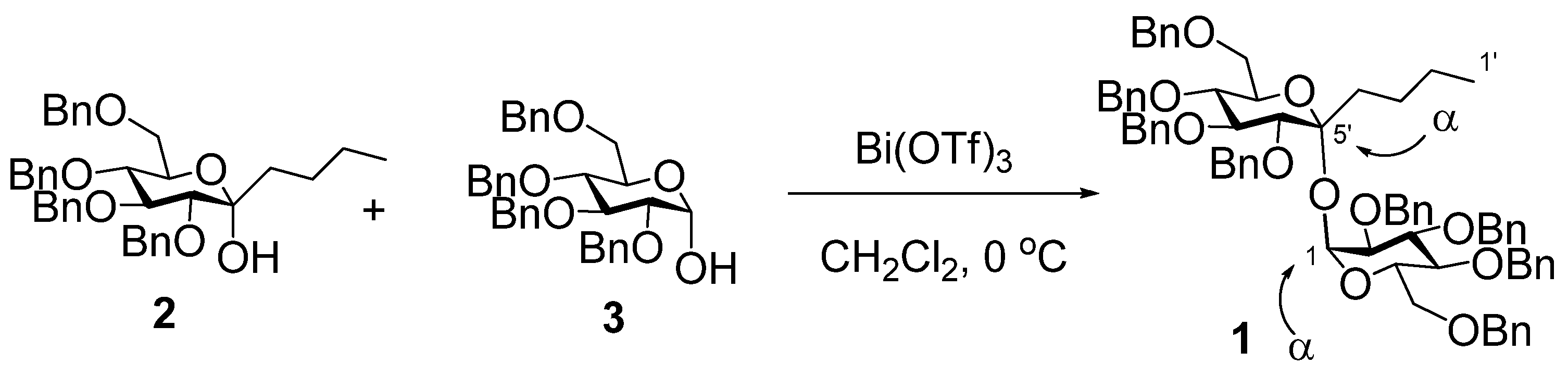
6,7,8,10-Tetra-O-benzyl-1,2,3,4-tetradeoxy-α-D-gluco-dec-5-ulopyranosyl 2,3,4,6-Tetra-O-benzyl-α-D-glucopyranoside
Abstract
:
Experimental
6,7,8,10-Tetra-O-benzyl-1,2,3,4-tetradeoxy-α-d-gluco-dec-5-ulopyranosyl 2,3,4,6-tetra-O-benzyl-α-d-glucopyranoside (1)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Yamanoi, T.; Matsuda, S. Reactions and uses of artificial ketoses. Heterocycles 2009, 79, 163–194 and references cited herein. [Google Scholar] [CrossRef]
- Benltifa, M.; Vidal, S.; Gueyrard, D.; Goekjian, P.G.; Msaddek, M.; Praly, J.P. 1,3-Dipolar cycloaddition reactions on carbohydrate-based templates: Synthesis of spiro-isoxazolines and 1,2,4-oxadiazoles as glycogen phosphorylase inhibitors. Tetrahedron Lett. 2006, 47, 6143–6147. [Google Scholar] [CrossRef]
- Pan, D.; Liu, J.; Senese, C.; Hopfinger, A.J.; Tseng, Y. Characterization of a ligand−receptor binding event using receptor-dependent four-dimensional quantitative structure−activity relationship analysis. J. Med. Chem. 2004, 47, 3075–3088. [Google Scholar] [CrossRef] [PubMed]
- Stolz, F.; Reiner, M.; Blume, A.; Reutter, W.; Schmidt, R.R. Novel UDP-glycal derivatives as transition state analogue inhibitors of UDP-GlcNAc 2-epimerase. J. Org. Chem. 2004, 69, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Somsák, L.; Kovács, L.; Tóth, M.; Ősz, E.; Szilágyi, L.; Györgydeák, Z.; Dinya, Z.; Docsa, T.; Tóth, B.; Gergely, P. Synthesis of and a comparative study on the inhibition of muscle and liver glycogen phosphorylases by epimeric pairs of d-gluco- and d-xylopyranosylidene-spiro-(thio)hydantoins and N-(d-glucopyranosyl) amides. J. Med. Chem. 2001, 44, 2843–2848. [Google Scholar] [CrossRef]
- Namme, R.; Mitsugi, T.; Takahashi, H.; Ikegami, S. Synthesis of PI-88 analogue using novel O-glycosidation of exo-methylenesugars. Tetrahedron Lett. 2005, 46, 3033–3036. [Google Scholar] [CrossRef]
- Li, X.; Ohtake, H.; Takahashi, H.; Ikegami, S. Direct methylenation of partially benzyl-protected sugar lactones by dimethyltitanocene. Synlett 2001, 1885–1888. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamashita, K.; Hotta, T.; Hashimoto, T.; Kikuchi, M.; Nishiyama, H. Synthesis of spirocyclic C-arylglycosides and -ribosides by ruthenium-catalyzed cycloaddition. Chem. Asian J. 2007, 2, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- van Hooft, P.A.V.; Oualid, F.E.; Overkleeft, H.S.; van der Marel, G.A.; van Boom, J.H.; Leeuwenburgh, M.A. Synthesis and elaboration of functionalised carbohydrate-derived spiroketals. Org. Biomol. Chem. 2004, 2, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Takahashi, H.; Ohtake, H.; Shiro, M.; Ikegami, S. Stereoselective synthesis and structure elucidation of spiro-ketodisaccharides. Tetrahedoron 2001, 57, 8053–8066. [Google Scholar] [CrossRef]
- Czernecki, S.; Perlat, M.C. C-Glycosides. 9. Stereospecific synthesis of C-glycosidic spiroketal of the papulacandins. J. Org. Chem. 1991, 56, 6289–6292. [Google Scholar] [CrossRef]
- Hanessian, S.; Ugolini, A. Synthesis of a chiral 1,7-dioxaspiro[5,5]undecene. A model for the spiroacetal subunit of avermectin B1a. Carbohydr. Res. 1984, 130, 261–269. [Google Scholar] [CrossRef]
- Yamanoi, T.; Oda, Y.; Muraishi, H.; Matsuda, S. Synthesis of a novel d-glucose-conjugated 15-crown-5 ether with a spiro ketal structure. Molecules 2008, 13, 1840–1845. [Google Scholar] [CrossRef] [PubMed]
- Yamanoi, T.; Nara, Y.; Matsuda, S.; Oda, Y.; Yoshida, A.; Katsuraya, K.; Watanabe, M. Synthetic approach to exo-glycals from 1-C-vinyl-d-glycopyranose derivatives via an SN1’-substitution mechanism. Synlett 2007, 785–789. [Google Scholar] [CrossRef]
- Tomooka, K.; Nakamura, Y.; Nakai, T. [2,3]-Wittig Rearrangement using Glucose as a Chiral Auxiliary: Asymmetric Transmission from the Anomeric Center. Synlett 1995, 321–322. [Google Scholar] [CrossRef]
- Matsuda, S.; Matsumura, K.; Watanabe, M.; Yamanoi, T. Synthesis of a partially benzylated derivative of the anhydro-d-altro-heptulose found in Coriaria japonica A. Tetrahedron Lett. 2007, 48, 5807–5810. [Google Scholar] [CrossRef]
- Matsuda, S.; Yamanoi, T.; Watanabe, M. Syntheses of a partially benzylated derivative of the anhydro-d-altro-heptulose found in Coriaria japonica A and of its analogs. Tetrahedron 2008, 64, 8082–8088. [Google Scholar] [CrossRef]
- Lay, L.; Meldal, M.; Nicotra, F.; Panza, L.; Russo, G. Stereoselective synthesis of the C-analogue of β-d-glucopyranosyl serine. Chem. Commun. 1997, 1469–1470. [Google Scholar] [CrossRef]
- Yamanoi, T.; Inoue, R.; Matsuda, S.; Katsuraya, K.; Hamasaki, K. Synthesis of trehalose mimics by bismuth(III) triflate or bis(trifluoromethane)sulfonimide-catalyzed 1-C-methyl-d-hexopyranosylation. Tetrahedron-Asymmetry 2006, 17, 2914–2918. [Google Scholar] [CrossRef]
- A similar paper has been reported after our publication of ref. 19: Namme, R.; Mitsugi, T.; Takahashi, H.; Ikegami, S. Development of ketoside-type analogues of trehalose by using-stereoselective O-glycosidation of ketose. Eur. J. Org. Chem. 2007, 3758–3764. [Google Scholar]
- Nishizaki, Y.; Yoshizane, C.; Toshimori, Y.; Arai, N.; Akamatsu, S.; Hanaya, T.; Arai, S.; Ikeda, M.; Kurimoto, M. Disaccharide-trehalose inhibits bone resorption in ovariectomized mice. Nutrition Res. 2000, 20, 653–664. [Google Scholar] [CrossRef]
- Oda, Y.; Yamanoi, T. Trimethylsilyl trifluoromethanesulfonate catalyzed nucleophilic substitution to give C- and N-glucopyranosides derived from d-glucopyranose. Synthesis 2007, 3021–3031. [Google Scholar] [CrossRef]
- Xue, J.L.; Cecioni, S.; He, L.; Vidal, S.; Praly, J.P. Variations on the SnCl4 and CF3CO2Ag-promoted glycosidation of sugar acetates: A direct, versatile and apparently simple method with either α or β stereocontrol. Carbohydr. Res. 2009, 344, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- The formation of the α,α-glycosyl linkages corresponded to our former reaction using 3,4,5,7-tetra-O-benzyl-1-deoxy-α-d-gluco-hept-2-ulopyranose and 3. See ref.19. 2 was more promptly activated by a catalytic amount of Bi(OTf)3 than 3. Therefore, we supposed 2 worked as the glycosyl donor and 3 served as the glycosyl acceptor.
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yamanoi, T.; Inoue, R.; Oda, Y.; Hamasaki, K. 6,7,8,10-Tetra-O-benzyl-1,2,3,4-tetradeoxy-α-D-gluco-dec-5-ulopyranosyl 2,3,4,6-Tetra-O-benzyl-α-D-glucopyranoside. Molbank 2010, 2010, M671. https://doi.org/10.3390/M671
Yamanoi T, Inoue R, Oda Y, Hamasaki K. 6,7,8,10-Tetra-O-benzyl-1,2,3,4-tetradeoxy-α-D-gluco-dec-5-ulopyranosyl 2,3,4,6-Tetra-O-benzyl-α-D-glucopyranoside. Molbank. 2010; 2010(2):M671. https://doi.org/10.3390/M671
Chicago/Turabian StyleYamanoi, Takashi, Ryo Inoue, Yoshiki Oda, and Keita Hamasaki. 2010. "6,7,8,10-Tetra-O-benzyl-1,2,3,4-tetradeoxy-α-D-gluco-dec-5-ulopyranosyl 2,3,4,6-Tetra-O-benzyl-α-D-glucopyranoside" Molbank 2010, no. 2: M671. https://doi.org/10.3390/M671