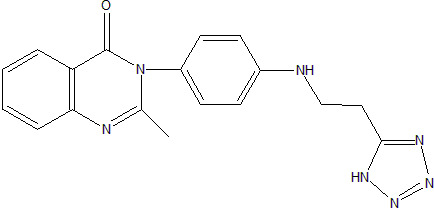
2-Methyl-3-{4-[2-(1H-tetrazol-5-yl)ethylamino]phenyl}-3H-quinazolin-4-one
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antimicrobial activity

3. Experimental
3.1. 2-Methyl-3-{4-[2-(1H-tetrazol-5-yl-ethylamino]phenyl}-3H-quinazolin-4-one (3)
3.2. Antimicrobial activity
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Kumar, A.; Tyagi, M.; Shrivastava, V.K. Newer potential quinazolinones as hypotensive agents. Indian J. Chem. 2003, 42B, 2142–2144. [Google Scholar] [CrossRef]
- Chandrika, P.M.; Yakaiah, T.; Rao, A.R.R.; Narsaiah, B.; ChakraReddy, N.; Sridhar, V.; Rao, J.V. Synthesis of novel 4,6-disubstituted Quinazoline derivatives, their anti-inflammatory and anti-cancer activity(cytotoxic) against U937 leukemia cell lines. Eur. J. Med. Chem. 2008, 43, 846–852. [Google Scholar]
- Shah, B.R.; Bhatt, J.J.; Patel, H.H.; Undavia, N.K.; Trivedi, P.B.; Desai, N.C. Synthesis of 2,3-disubstituted-3,1-quinazolin-4(4H)-ones as potential anticancer and anti-HIV agents. Indian J. Chem. 1995, 34B, 201–208. [Google Scholar] [CrossRef]
- Rani, P.; Archana; Srivastava, V.K.; Kumar, A. Synthesis and anti-inflammatory activity of some new 2,3-disubstituted-6-monosubstituted-quinazolin-4(3H)-ones. Ind. J. Heterocycl. Chem. 2002, 41B, 2642–2646. [Google Scholar]
- Alagarsamy, V.; Muthukumar, V.; Pavalarani, N.; Vasanthanathan, P.; Revathi, R. Synthesis, Analgesic and Anti-inflammatory Activities of Some Novel 2,3-Disubstituted Quinazoline-4(3H)-ones. Biol. Pharm. Bull. 2003, 26, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Sarah, T.; Zehra, T. Thiadiazolyl quinazoolones as potential antiviral and antihypertensive agents. Indian J. Chem. 2004, 43B, 180–183. [Google Scholar]
- Joshi, V.; Chaudhari, R.P. Synthesis of Some New 4-Quinazolnone-2-carboxy Esters, 2-Carboxamides, 2-Carboxyhydrazides & their Tosyl Derivatives Having Potential Biological Activity. Indian J. Chem. 1987, 28B, 602–604. [Google Scholar]
- Wasfy, A.A.F. Studies on quinazolines: Part II-Synthesis and antimicrobial evaluation of some 2,2-disubstituted-3,3-biquinazoin-4(3H)-ones. Indian J. Chem. 2003, 42B, 3102–3107. [Google Scholar] [CrossRef]
- Nanda, A.K.; Ganguli, S.; Chakraborty, R. Antibacterial Activity of some 3-(Arylideneamino)-2-phenylquinazoline-4-(3H)-nes Synthesis and Preliminary QSAR Studies. Molecules 2007, 12, 2413–2426. [Google Scholar] [CrossRef] [PubMed]
- Shivram Holla, B.; Padmaja, M.T.; Shivnanda, M.K.; Akbarali, P.M. Synthesis and antibacterial activity of nitro-furylvinylquinazolinones. Indian J. Chem. 1998, 37B, 715–716. [Google Scholar]
- Butler, R.N. The Structure, Reactions Synthesis and Uses of Heterocyclic Compounds. In Comprehensive Heterocyclic Chemistry; Potts, K.T., Ed.; Pregamon Press: Oxford, UK, 1984; pp. 791–838. [Google Scholar]
- Butler, R.N. The Structure, Reactions, Synthesis and Uses of Heterocyclic Compounds. In Comprehensive Heterocyclic Chemistry-II; Storr, R.C., Ed.; Pregamon Press: Oxford, UK, 1996; pp. 621–678. [Google Scholar]
- Singh, H.; Chawla, A.S.; Kapoor, U.K.; Paul, D.; Malhotra, R.K. 4-Medicinal Chemistry of Tetrazole. Prog. Med. Chem. 1980, 17, 151–183. [Google Scholar] [PubMed]
- Bradbury, R.H.; Allot, C.P.; Dennis, M.; Girdwood, J.A.; Kenny, P.W.; Major, J.S.; Oldham, A.A.; Ratccliffe, A.H.; Rivett, J.E.; Roberts, D.A.; Robins, P.J. New nonpeptide angiotensine II receptorantagonists.3. Syntheis, biological properties, and structure-activity relationships of 2-alkyl-4-(biphenylmethoxy)pyridine derivatives. J. Med. Chem. 1993, 36, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Upadhayaya, R.S.; Jain, S.; Sinha, N.; Kishore, N.; Chandra, R.; Arora, S.K. Synthesis of novel substituted tetrazoles having antifungal activity. Eur. J. Med. Chem. 2004, 39, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, B.R.; Kavitha, H.P. Synthesis of 4-[10H-phenothiazine-10-yl(1H—tetrazol-5yl)-methyl]phenol. Molbank 2009, 2009, M621. [Google Scholar] [CrossRef]
- Errede, L.A.; McBrady, J.J.; Oien, H.T. Acylanthranils. 2. The Problem of Selectivity in the Reaction of Acetylanthranil with Anilines. J. Org. Chem. 1976, 41, 1765–1768. [Google Scholar] [CrossRef]
| Compound | Zone of inhibition (mm) | |||
|---|---|---|---|---|
| S. aureus | E. Coli | C. albicans | A. niger | |
| Compound 3 | 11 | 13 | 8 | 11 |
| Control | - | - | - | - |
| Standard | 40 | 41 | 23 | 22 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Arulmurugan, S.; Kavitha, H.P. 2-Methyl-3-{4-[2-(1H-tetrazol-5-yl)ethylamino]phenyl}-3H-quinazolin-4-one. Molbank 2010, 2010, M695. https://doi.org/10.3390/M695
Arulmurugan S, Kavitha HP. 2-Methyl-3-{4-[2-(1H-tetrazol-5-yl)ethylamino]phenyl}-3H-quinazolin-4-one. Molbank. 2010; 2010(3):M695. https://doi.org/10.3390/M695
Chicago/Turabian StyleArulmurugan, Subramaniyan, and Helen P. Kavitha. 2010. "2-Methyl-3-{4-[2-(1H-tetrazol-5-yl)ethylamino]phenyl}-3H-quinazolin-4-one" Molbank 2010, no. 3: M695. https://doi.org/10.3390/M695