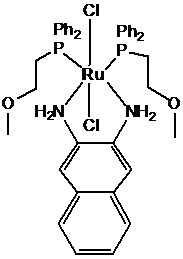
Trans-dichloro-2,3-naphthalenediamine bis[(2-methoxyethyl)(diphenyl)phosphine]ruthenium(II) Complex
Abstract
:1. Introduction
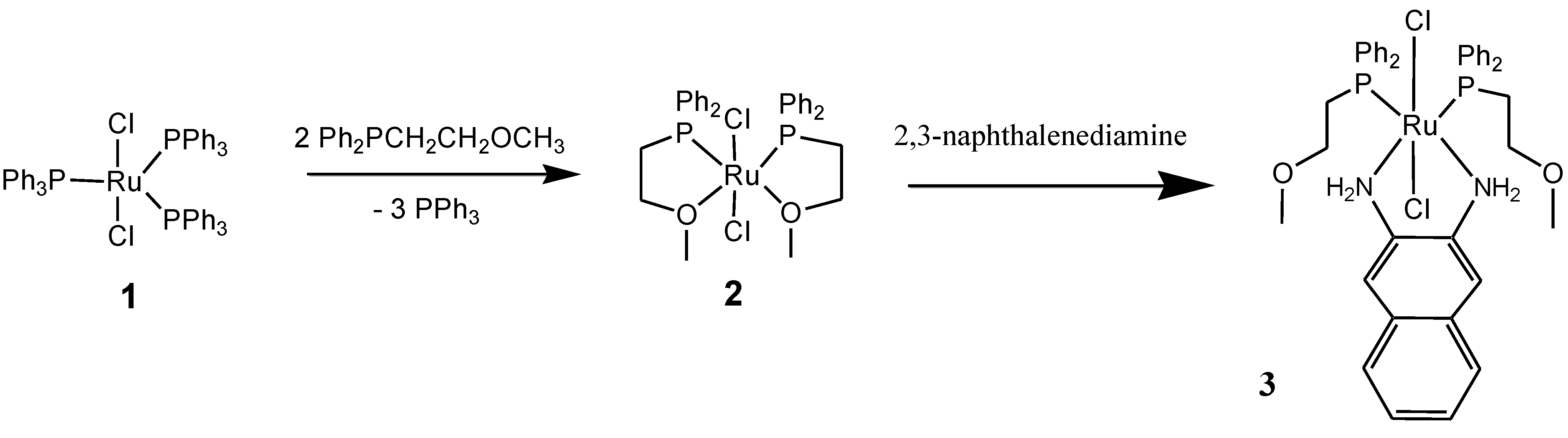
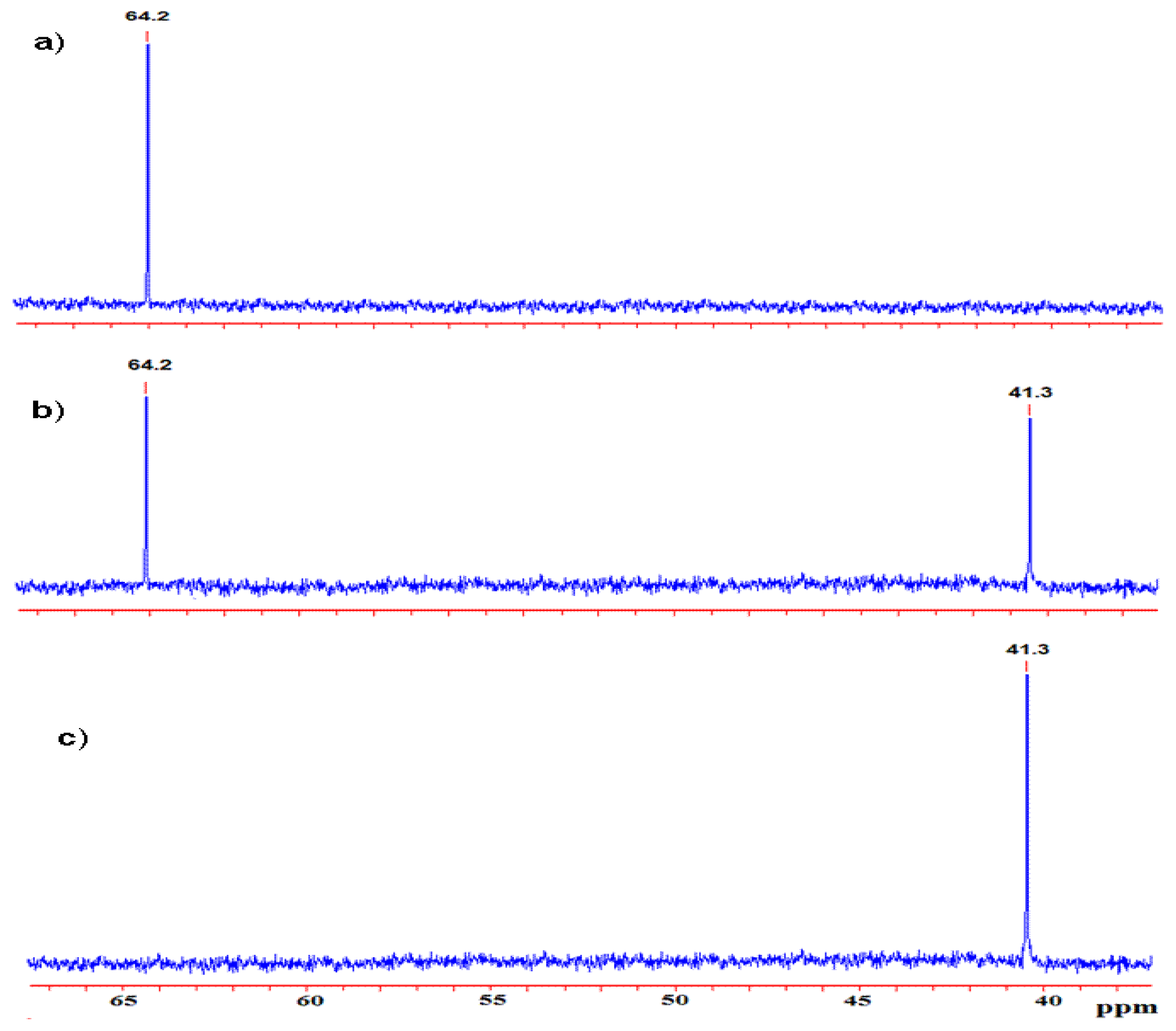
2. Result and Discussion
3. Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Warad, I. Supported and Non-Supported Ruthenium(II)/Phosphine/[3-(2-Aminoethyl)aminopropyl]trimethoxysilane Complexes and Their Activities in the Chemoselective Hydrogenation of trans-4-Phenyl-3-butene-2-al. Molecules 2010, 15, 4652–4669. [Google Scholar] [CrossRef] [PubMed]
- Lindner, E.; Warad, I.; Eichele, K.; Mayer, H.A. Synthesis and Structures of an Array of Diamine(ether-phosphine)ruthenium(II) Complexes and Their Application in the Catalytic Hydrogenation of trans-4-phenyl-3-butene-2-one. Inorg. Chim. Acta 2003, 350, 49–56. [Google Scholar] [CrossRef]
- Lu, Z.-L.; Eichele, K.; Warad, I.; Mayer, H.A.; Lindner, E.; Jiang, Z.; Schurig, V. Supported Organometallic Complexes. XXXVIII Bis(methoxyethyldimethylphosphine)ruthenium(II) Complexes as Transfer Hydrogenation Catalysts. Z. Anorg. Allg. Chem. 2003, 629, 1308–1315. [Google Scholar] [CrossRef]
- Warad, I.; Lindner, E.; Eichele, K.; Mayer, A.H. Cationic Diamine(ether–phosphine)ruthenium(II) Complexes as Precursors for the Hydrogenation of trans-4-phenyl-3-butene-2-one. Inorg. Chim. Acta 2004, 357, 1847–1853. [Google Scholar] [CrossRef]
- Lindner, E.; Ghanem, A.; Warad, I.; Eichele, K.; Mayer, H.A.; Schurig, V. Asymmetric Hydrogenation of an Unsaturated Ketone by Diamine(ether–phosphine)ruthenium(II) Complexes and Lipase-Catalyzed Kinetic Resolution: A Consecutive Approach. Tetrahedron Asymmetry 2003, 14, 1045–1050. [Google Scholar]
- Lindner, E.; Al-Gharabli, S.; Warad, I.; Mayer, H.A.; Steinbrecher, S.; Plies, E.; Seiler, M.; Bertagnolli, H. Diaminediphosphineruthenium(II) Interphase Catalysts for the Hydrogenation of α,ß-Unsaturated Ketones. Z. Anorg. Allg. Chem. 2003, 629, 161–171. [Google Scholar] [CrossRef]
- Lu, Z.-L.; Eichele, K.; Warad, I.; Mayer, H.A.; Lindner, E.; Jiang, Z.; Schurig, V. Bis(methoxyethyldimethylphosphine)ruthenium(II) Complexes as Transfer Hydrogenation Catalysts. Z. Anorg. Allg. Chem. 2003, 629, 1308–1315. [Google Scholar] [CrossRef]
- Warad, I.; Al-Resayes, S.; Eichele, E. Crystal Structure of trans-Dichloro-1,3-propanediamine-bis-[(2-methoxyethyl)diphenylphosphine]ruthenium(II), RuCl2(C3H10N2)(C15H17OP)2. Z. Kristallogr. NCS 2006, 221, 275–277. [Google Scholar]
- Warad, I. Synthesis and Crystal Structure of cis-Dichloro-1,2-ethylenediamine-bis[1,4-(diphenylphosphino)butane]ruthenium(II) Dichloromethane Disolvate, RuCl2(C2H8N2)(C28H28P2)-2CH2Cl2. Z. Kristallogr. NCS 2007, 222, 415–417. [Google Scholar]
- Warad, I.; Siddiqui, M.; Al-Resayes, S.; Al-Warthan, A.; Mahfouz, R. Synthesis, Characterization, Crystal Structure and Chemical Behavior of [1,1-Bis(diphenylphosphinomethyl)ethene]ruthenium (II) Complex Toward Primary Alkylamine Addition. Trans. Met. Chem. 2009, 34, 347–354. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Warad, I. Trans-dichloro-2,3-naphthalenediamine bis[(2-methoxyethyl)(diphenyl)phosphine]ruthenium(II) Complex. Molbank 2010, 2010, M696. https://doi.org/10.3390/M696
Warad I. Trans-dichloro-2,3-naphthalenediamine bis[(2-methoxyethyl)(diphenyl)phosphine]ruthenium(II) Complex. Molbank. 2010; 2010(3):M696. https://doi.org/10.3390/M696
Chicago/Turabian StyleWarad, Ismail. 2010. "Trans-dichloro-2,3-naphthalenediamine bis[(2-methoxyethyl)(diphenyl)phosphine]ruthenium(II) Complex" Molbank 2010, no. 3: M696. https://doi.org/10.3390/M696