1,1,2,2-Tetrakis[(benzoylamino)methyl]hydrazine
Abstract
:1. Introduction
2. Results and Discussion
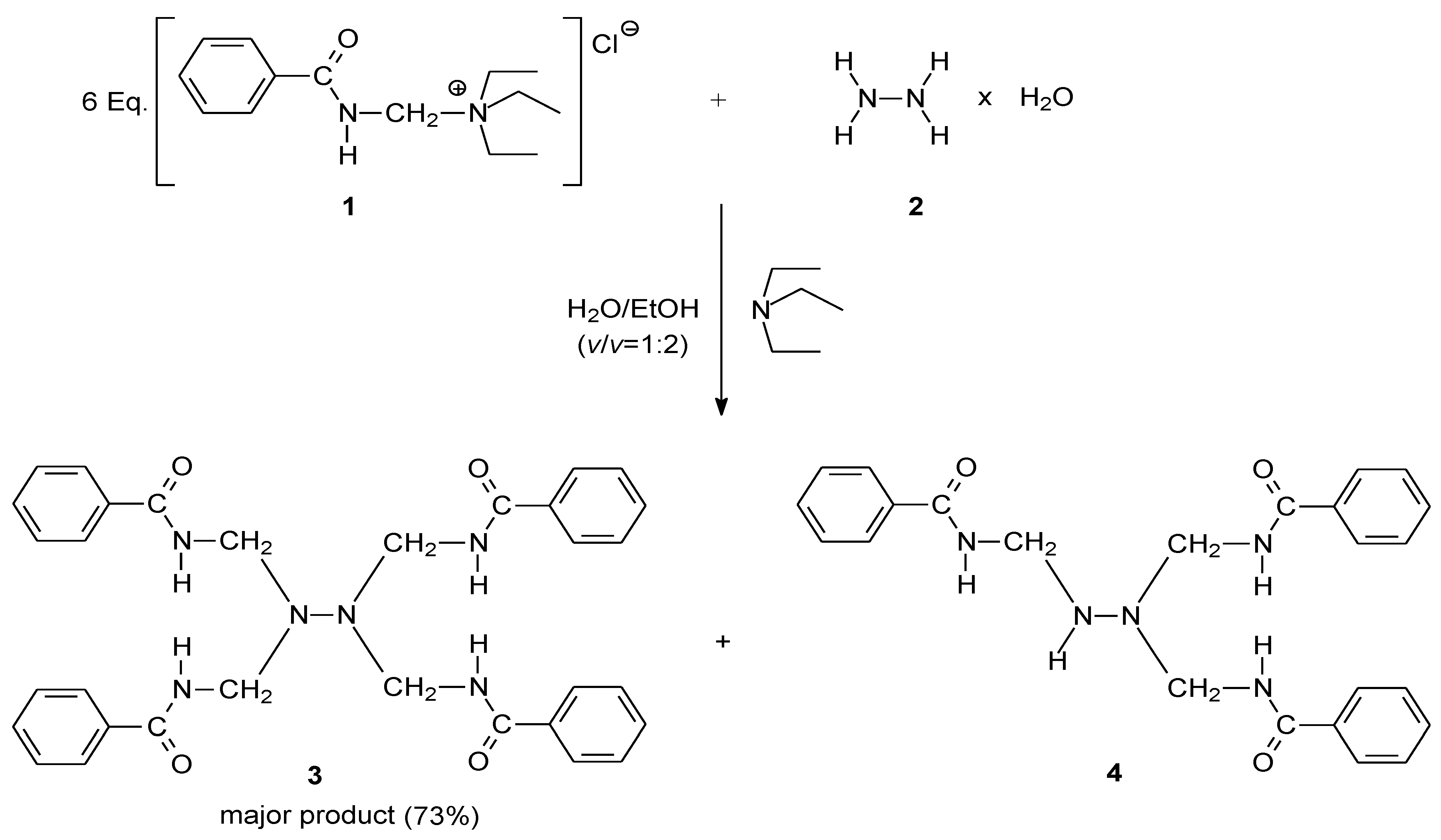
3. Experimental
3.1. Synthesis of 1,1,2,2-tetrakis[(benzoylamino)methyl]hydrazine (3)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
References
- Tiwari, K.A.; Singh, K.V.; Bajpai, A.; Shukla, G.; Singh, S.; Mishra, K.A. Synthesis and biological properties of 4-(3H)-quinazolone derivatives. Eur. J. Med. Chem. 2007, 42, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Mai, A.; Artico, M.; Valente, S.; Cerbara, I.; Befani, O.; Turini, P.; Vedova, D.L.; Agostinelli, E. Synthesis and biochemical evaluation of (R)-5-acyloxymethyl- and (S)-5-acylaminomethyl-3-(1H-pyrrol-1-yl)-2-oxazolidinones as new anti-monoamine oxidase (anti-MAO) agents. ARKIVOC 2004, 2004, 32–43. [Google Scholar]
- Zlotin, G.S.; Sharova, V.I.; Luk`yanov, A.O. Chemical properties of N-(amidomethy)- and N-(imidomethyl)glycine derivatives 2. Reactions at alkoxycarbonyl and carboxyl groups. Rus. Chem. Bull. 1996, 45, 1680–1687. [Google Scholar] [CrossRef]
- Schioppacassi, G.; Morvillo, E.; Bruna, C.D.; Franceschi, G.; Foglio, M. In vitro and in vivo evaluation of benzamidomethyl-benzylpenicillinate (FI7303). A new 'repository' form. Chemotherapy 1978, 24, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, H.; Nielsen, N.M.; Buur, A. Aspirin prodrugs: Synthesis and hydrolysis of 2-acetoxybenzoate esters of various N-(hydroxyalkyl) amides. Int. J. Pharm. 1988, 44, 151–158. [Google Scholar] [CrossRef]
- Moreira, R.; Calheiros, T.; Cabrita, J.; Mendes, E.; Pimentel, M.; Iley, J. Acyloxymethyl as a drug protecting group. Part 3. Tertiary O-Amidomethyl esters of penicillin G: Chemical hydrolysis and anti-bacterial activity. Pharm. Res. 1996, 13, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Getz, J.J.; Prankerd, R.J.; Sloan, K.B. Mechanism of hydrolysis of benzamidomethyl derivatives of phenols and its implications for prodrug design. J. Org. Chem. 1992, 57, 1702–1706. [Google Scholar] [CrossRef]
- Ragnarsson, U. Synthetic methodology for alkyl substituted hydrazines. Chem. Soc. Rev. 2001, 30, 205–213. [Google Scholar] [CrossRef]
- Ling, L.; Urichuk, L.J.; Sloley, B.D.; Coutts, R.T.; Baker, G.B.; Shan, J.J.; Pang, P.K.T. Synthesis of N-propargylphenelzine and analogues as neuroprotective agents. Bioorg. Med. Chem. Lett. 2001, 11, 2715–2717. [Google Scholar] [CrossRef]
- Rabong, C.; Jordis, U.; Phopase, J.B. NXO Building Blocks for Backbone Modification of Peptides and Preparation of Pseudopeptides. J. Org. Chem. 2010, 75, 2492–2500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Durkin, J.P.; Windsor, W.T. Azapeptides as inhibitors of the hepatitis C virus NS3 serine protease. Bioorg. Med. Chem. Lett. 2002, 12, 1005–1008. [Google Scholar] [CrossRef]
- Raja, A.; Lebbos, J.; Kirkpatrick, P. Fresh from the Pipeline: Atazanavir sulphate. Nat. Rev. Drug Discov. 2003, 2, 857–858. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Cherney, M.M.; Huitema, C.; Liu, J.; James, K.E.; Powers, J.C.; Eltis, L.D.; James, M.N.G. Crystal structures of the main peptidase from the SARS coronavirus inhibited by a substrate-like aza-peptide epoxide. J. Mol. Biol. 2005, 353, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Clennan, E.L.; Noe, L.J.; Wen, T. Hydrazines: New charge-transfer physical quenchers of singlet oxygen. J. Am. Chem. Soc. 1990, 112, 5080–5085. [Google Scholar] [CrossRef]
- Nelsen, S.F.; Rumack, D.T.; Meot-Ner, M. Kinetic effects of an unusually large neutral to radical cation geometry change. Slow electron-transfer reactions between alkylhydrazines. J. Am. Chem. Soc. 1987, 109, 1373–1379. [Google Scholar] [CrossRef]
- Nelsen, S.F.; Rumack, D.T.; Meot-Ner, M. Gas-phase electron-transfer equilibrium studies on tetraalkylhydrazines: Geometry effects on ionization thermochemistry, relaxation energies, and ion solvation energies. J. Am. Chem. Soc. 1988, 110, 7945–7952. [Google Scholar] [CrossRef]
- Nelsen, S.F.; Peacock, V.; Weisman, G.R. Single-electron oxidation equilibriums of tetraalkylhydrazines. Comparison of solution E0 values and vapor-phase ionization potentials. J. Am. Chem. Soc. 1976, 98, 5269–5277. [Google Scholar] [CrossRef]
- Nelsen, S.F.; Hintz, P.J. Electrochemical oxidation of tetraalkylhydrazines. Effects of hydrazine and hydrazine radical cation geometry. J. Am. Chem. Soc. 1972, 94, 7108–7113. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Benzamidomethylation with (benzamidomethyl)triethylammonium chloride. 2. A simple method for benzamidomethylation of thiols, amines and carboxylic acods. Molecules 2000, 5, 927–936. [Google Scholar] [CrossRef]
- Popovski, E. Synthesis of N-(N’-benoylhydrazinomethyl)benzamide. Molbank 2007, 2007, M525. [Google Scholar] [CrossRef]
- Popovski, E. Synthesis of N-[N’-(2-hydroxy-2,2-diphenylacethyl)hydrazinomethyl]benzamide. Molbank 2007, 2007, M526. [Google Scholar] [CrossRef]
- Tanatarec, J.; Mikova, B.; Popovski, E. Tribenzamidomethyl hydrazine. Molbank 2010, 2010, M710. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Simple Method for Benzamidomethylation of Phenols in Water Solution. Synth. Commun. 1999, 29, 3451–3458. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tanatarec, J.; Mikhova, B.; Popovski, E. 1,1,2,2-Tetrakis[(benzoylamino)methyl]hydrazine. Molbank 2011, 2011, M722. https://doi.org/10.3390/M722
Tanatarec J, Mikhova B, Popovski E. 1,1,2,2-Tetrakis[(benzoylamino)methyl]hydrazine. Molbank. 2011; 2011(1):M722. https://doi.org/10.3390/M722
Chicago/Turabian StyleTanatarec, Jasmina, Bozhana Mikhova, and Emil Popovski. 2011. "1,1,2,2-Tetrakis[(benzoylamino)methyl]hydrazine" Molbank 2011, no. 1: M722. https://doi.org/10.3390/M722



