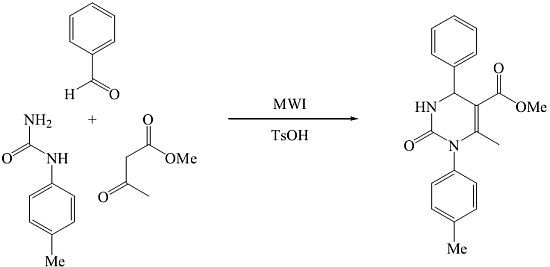
Methyl 6-Methyl-1-(4-methylphenyl)-2-oxo-4-phenyl-1,2,3,4-tetrahydropyrimidine-5-carboxylate
Abstract
:Experimental Procedure
Structural Characterization
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References
- Kappe, C.O. 100 Years of Biginelli dihydropyrimidine synthesis. Tetrahedron 1993, 49, 6937–6963. [Google Scholar] [CrossRef]
- Kappe, C.O. Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog. Acc. Chem. Res. 2000, 33, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Biginelli, P. Aldehyde−urea derivatives of aceto- and oxaloacetic acids. Gazz. Chim. Ital. 1893, 23, 360–413. [Google Scholar]
- Li, Y.X.; Bao, W.L. Microwave-assisted Solventless Biginelli Reaction Catalyzed by Montmorillonite Clay-SmCl3. 6H2O System. Chin. Chem. Lett. 2003, 14, 993–995. [Google Scholar]
- Yadav, J.S.; Reddy, B.V.; Reddy, E.J.; Ramalingam, T. Microwave-assisted efficient synthesis of dihydropyrimidines: improved high yielding protocol for the Biginelli reaction. J. Chem. Res. (S) 2000, 7, 354–355. [Google Scholar] [CrossRef]
- Stefani, H.A.; Gatti, P.M. 3,4-Dihydropyrimidin-2(1H)-Ones: fast synthesis under microwave irradiation in solvent free conditions. Synth. Commun. 2000, 30, 2165–2173. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, K.B. Ultrasound-accelerated synthesis of 3,4-dihydropyrimidin-2(1H)-ones with ceric ammonium nitrate. J. Chem. Soc. 2001, 16, 1939–1941. [Google Scholar] [CrossRef]
- Li, J.T.; Han, J.F.; Yang, J.H.; Li, T.S. An efficient synthesis of 3,4-dihydropyrimidin-2-ones catalyzed by NH2SO3H under ultrasound irradiation. Ultrason. Sonochem. 2003, 10, 119–122. [Google Scholar] [CrossRef]
- Peng, J.J.; Deng, Y.Q. Ionic liquids catalyzed Biginelli reaction under solvent-free conditions. Tetrahedron Lett. 2001, 42, 5917–5919. [Google Scholar] [CrossRef]
- Shao, G.Q. Biginelli Condensation Assisted by Microwave Irradiation in Ionic Liquids. Chin. J. Synth. Chem. 2004, 12, 325–328. [Google Scholar]
- Niralwad, K.S.; Shingate, B.B.; Shingare, M.S. Ultrasound-assisted One-Pot Synthesis of Octahydroquinazolinone Derivatives Catalyzed by Acidic Ionic Liquid [tbmim]Cl2/AlCl3. J. Chin. Chem. Soc. 2010, 57, 89–92. [Google Scholar] [CrossRef]
- Cai, Y.F.; Yang, H.M.; Li, L.; Jiang, K.Z.; Lai, G.Q.; Jiang, J.X.; Xu, L.W. Cooperative and Enantioselective NbCl5/Primary Amine Catalyzed Biginelli Reaction. Eur. J. Org. Chem. 2010, 26, 4986–4990. [Google Scholar] [CrossRef]
- Hu, E.H.; Sidler, D.R.; Dolling, U.H. Unprecedented Catalytic Three Component One-Pot Condensation Reaction: An Efficient Synthesis of 5-Alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones. J. Org. Chem. 1998, 63, 3454–3457. [Google Scholar] [CrossRef]
- Cheng, J.; Qi, D.Y. An efficient and solvent-free one-pot synthesis of dihydropyrimidinones under microwave irradiation. Chin. Chem. Lett. 2007, 18, 647–650. [Google Scholar]
- Ma, Y.; Qian, C.T.; Wang, L.M.; Yang, M. Lanthanide triflate catalyzed Biginelli reaction. One-pot synthesis of dihydropyrimidinones under solvent-free conditions. J. Org. Chem. 2000, 65, 3864–3868. [Google Scholar] [CrossRef] [PubMed]
- Ranu, B.C.; Hajra, A.; Jana, U. Indium(III) Chloride Catalyzed One-Pot Synthesis of Dihydropyrimidinones by a Three-Component Coupling of 1,3-Dicarbonyl Compounds, Aldehydes, and Urea: An Improved Procedure for the Biginelli Reaction. J. Org. Chem. 2000, 65, 6270–6272. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, A.; Bazgir, A.; Teimouri, F. Ammonium chloride-catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2-(1H)-ones under solvent-free conditions. Tetrahedron Lett. 2003, 44, 857–859. [Google Scholar] [CrossRef]
- Paraskar, A.S.; Dewkar, G.K.; Sudalai, A. Cu(OTf)2: A reusable catalyst for high-yield synthesis of 3, 4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett. 2003, 44, 3305–3308. [Google Scholar] [CrossRef]
- Lei, M.; Ma, L.; Hu, L.H. Cu(ClO4)2·6H2O as an Efficient Catalyst for the Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones Under Solvent-Free Conditions. Synth. Commun. 2011, 41, 3071–3077. [Google Scholar] [CrossRef]
- Dondoni, A.; Massi, A. Design and Synthesis of New Classes of Heterocyclic C-Glycoconjugates and Carbon-Linked Sugar and Heterocyclic Amino Acids by Asymmetric Multicomponent Reactions (AMCRs). Acc. Chem. Res. 2006, 39, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Shi, F.; Gong, L.-Z. Brφnsted-Acid-Catalyzed Asymmetric Multicomponent Reactions for the Facile Synthesis of Highly Enantioenriched Structurally Diverse Nitrogenous Heterocycles. Acc. Chem. Res. 2011, 44, 1156–1171. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Moorthy, J.N. Enantioselective Organocatalytic Biginelli Reaction: Dependence of the Catalyst on Sterics, Hydrogen Bonding, and Reinforced Chirality. J. Org. Chem. 2011, 76, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, J.S.; Luo, S.-W.; Fan, W.; Gong, L.-Z. Highly Enantioselective Organocatalytic Biginelli and Biginelli-Like Condensations: Reversal of the Stereochemistry by Tuning the 3,3′-Disubstituents of Phosphoric Acids. J. Am. Chem. Soc. 2010, 132, 10953–10953. [Google Scholar] [CrossRef]
- Li, N.; Chen, X.-H.; Song, J.; Luo, S.-W.; Fan, W.; Gong, L.-Z. Highly Enantioselective Organocatalytic Biginelli and Biginelli-Like Condensations: Reversal of the Stereochemistry by Tuning the 3,3′-Disubstituents of Phosphoric Acids. J. Am. Chem. Soc. 2009, 131, 15301–15310. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-L.; Xu, X.-P.; Ji, S.-J. Brφnsted Base-Catalyzed One-Pot Three-Component Biginelli-Type Reaction: An Efficient Synthesis of 4,5,6-Triaryl-3,4-dihydropyrimidin-2(1H)-one and Mechanistic Study. J. Org. Chem. 2010, 75, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Nandi, G.C.; Samai, S.; Singh, M.S. Biginelli and Hantzsch-Type Reactions Leading to Highly Functionalized Dihydropyrimidinone, Thiocoumarin, and Pyridopyrimidinone Frameworks via Ring Annulation with β-Oxodithioesters. J. Org. Chem. 2010, 75, 7785–7795. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Dai, P.; Schaus, S.E. Asymmetric Mannich Reaction of Dicarbonyl Compounds with α-Amido Sulfones Catalyzed by Cinchona Alkaloids and Synthesis of Chiral Dihydropyrimidones. J. Org. Chem. 2007, 72, 9998–10008. [Google Scholar] [CrossRef] [PubMed]
- Goss, J.M.; Schaus, S.E. Enantioselective Synthesis of SNAP-7941: Chiral Dihydropyrimidone Inhibitor of MCH1-R. J. Org. Chem. 2008, 73, 7651–7656. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Singh, S. Chemical resolution of inherently racemic dihydropyrimidinones via a site selective functionalization of Biginelli compounds with chiral electrophiles: A case study. Tetrahedron 2009, 65, 4106–4112. [Google Scholar] [CrossRef]
- Liu, X.; Lin, L.; Feng, X. Amide-based bifunctional organocatalysts in asymmetric reactions. Chem. Commun. 2009, 6145–6158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, J.; Miao, J.; Chen, R. Bifunctional primary amine-thiourea–TfOH (BPAT·TfOH) as a chiral phase-transfer catalyst: the asymmetric synthesis of dihydropyrimidines. Org. Biomol. Chem. 2011, 9, 3050–2054. [Google Scholar] [CrossRef] [PubMed]
- Isambert, N.; Duque, M.M.S.; Plaquevent, J.C.; Genisson, Y.; Rodriguez, J.; Constantieux, T. Multicomponent reactions and ionic liquids: a perfect synergy for eco-compatible heterocyclic synthesis. Chem. Soc. Rev. 2011, 40, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-H.; Xu, X.-Y.; Liu, H.; Cun, L.-F.; Gong, L.-Z. Highly Enantioselective Organocatalytic Biginelli Reaction. J. Am. Chem. Soc. 2006, 128, 14802–14803. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, F.; Zhu, C. Highly Enantioseletive Biginelli Reaction Using a New Chiral Ytterbium Catalyst: Asymmetric Synthesis of Dihydropyrimidines. J. Am. Chem. Soc. 2005, 127, 16386–16387. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Chai, Z.; Liu, X.-Y.; Zhao, G.; Wang, S.-W. Synthesis of Substituted 5-(Pyrrolidin-2-yl)tetrazoles and Their Application in the Asymmetric Biginelli Reaction. Eur. J. Org. Chem. 2009, 904–911. [Google Scholar] [CrossRef]
- Goss, J.M.; Schaus, S.E. Enantioselective Synthesis of SNAP-7941: Chiral Dihydropyrimidone Inhibitor of MCH1-R. J. Org. Chem. 2008, 73, 7651–7656. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Chai, Z.; Liu, X.-Y.; Zhao, G.; Wang, S.-W. Synthesis of Substituted 5-(Pyrrolidin-2-yl)tetrazoles and Their Application in the Asymmetric Biginelli Reaction. Eur. J. Org. Chem. 2009, 904–911. [Google Scholar] [CrossRef]
- Salehi, H.; Guo, Q.X. Efficient Magnesium Bromide-Catalyzed One-pot Synthesis of Substituted 1,2,3,4-Tetrahydropyrimidin-2-ones Under Solvent-free Conditions. Chin. J. Chem. 2005, 23, 91–97. [Google Scholar] [CrossRef]
- Ryabukhin, S.V.; Plaskon, A.S.; Bondarenko, S.S.; Ostapchuk, E.N.; Grygorenko, O.O.; Shishkin, O.V.; Tolmachev, A.A. Acyl pyruvates as synthons in the Biginelli reaction. Tetrahedron Lett. 2010, 51, 4229–4232. [Google Scholar] [CrossRef]
- de la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Stadler, A.; Kappe, C.O. Automated Library Generation Using Sequential Microwave-Assisted Chemistry. Application toward the Biginelli Multicomponent Condensation. J. Comb. Chem. 2001, 3, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.A.; Strauss, C.R. Toward Rapid, “Green”, Predictable Microwave-Assisted Synthesis. Acc. Chem. Res. 2005, 38, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Petit, A.; Hamelin, J.; Texier-Boullet, F.; Jacquault, P.; Mathe, D. New Solvent-Free Organic Synthesis Using Focused Microwaves. Synthesis 1998, 9, 1213–1234. [Google Scholar] [CrossRef]
- Vanna, R.S. Solvent-free organic syntheses. Green Chem. 1999, 43. [Google Scholar]
- Xue, S; Shen, Y.-C.; Li, Y.-L.; Shen, X.-M.; Guo, Q.-X. Synthesis of 4-Aryl-3, 4-dihydropyrimidinones Using Microwaveassisted Solventless Biginelli Reaction. Chin. J. Chem. 2002, 20, 385–289. [Google Scholar]
- Jiang, C.; You, Q.D. An efficient and solvent-free one-pot synthesis of dihydropyrimidinones under microwave irradiation. Chin. Chem. Lett. 2007, 18, 647–650. [Google Scholar] [CrossRef]
- Zhan, H.W.; Wang, J.X.; Wang, X.T. Solvent- and catalyst-free synthesis of dihydropyrimidinthiones in one-pot under focused microwave irradiation conditions. Chin. Chem. Lett. 2008, 19, 1183–1185. [Google Scholar] [CrossRef]
- Polshettiway, V.; Varma, R.S. Microwave-Assisted Organic Synthesis and Transformations using Benign Reaction Media. Acc. Chem. Res. 2008, 41, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Caddick, S.; Fitzmaurice, R. Microwave enhanced synthesis. Tetrahedron 2009, 65, 3325–3355. [Google Scholar] [CrossRef]
- Fang, Z.; Lam, Y. A rapid and convenient synthesis of 5-unsubstituted 3,4-dihydropyrimidin-2-ones and thiones. Tetrahedron 2011, 67, 1294–1297. [Google Scholar] [CrossRef]
- Sharma, A.; Appukkuttana, P.; Van der Eycken, E. Microwave-assisted synthesis of medium-sized heterocycles. Chem. Commun. 2012, 48, 1623–1637. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, Q.; Liu, Q.; Wang, H. Methyl 6-Methyl-1-(4-methylphenyl)-2-oxo-4-phenyl-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank 2012, 2012, M752. https://doi.org/10.3390/M752
Chen Q, Liu Q, Wang H. Methyl 6-Methyl-1-(4-methylphenyl)-2-oxo-4-phenyl-1,2,3,4-tetrahydropyrimidine-5-carboxylate. Molbank. 2012; 2012(2):M752. https://doi.org/10.3390/M752
Chicago/Turabian StyleChen, Qing, Qingjian Liu, and Haiping Wang. 2012. "Methyl 6-Methyl-1-(4-methylphenyl)-2-oxo-4-phenyl-1,2,3,4-tetrahydropyrimidine-5-carboxylate" Molbank 2012, no. 2: M752. https://doi.org/10.3390/M752